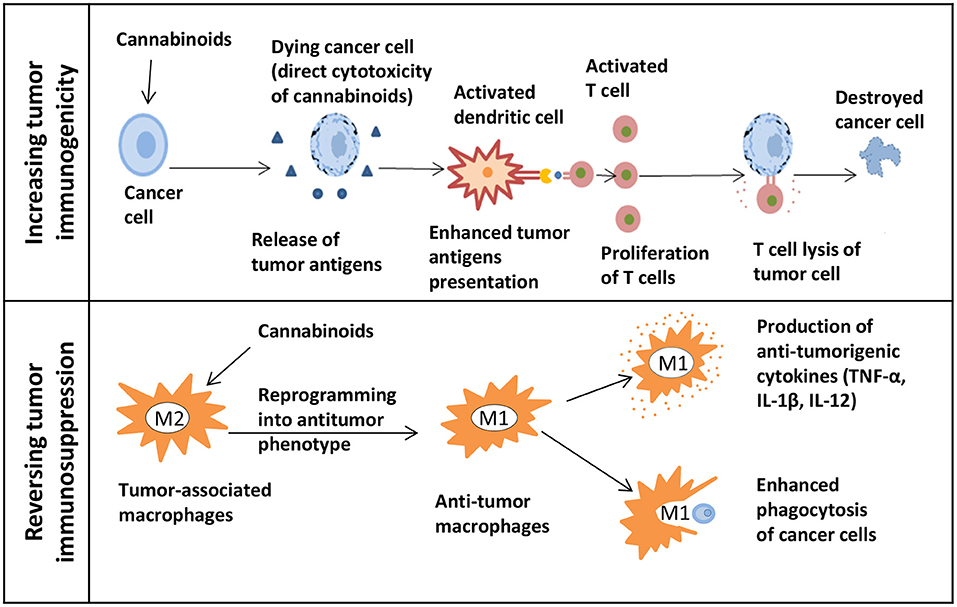
“Purified cannabinoids have been shown to prevent proliferation and induce apoptosis in colorectal carcinoma cell lines.
To assess the cytotoxic effect of cannabinoid extracts and purified cannabinoids on both colorectal polyps and normal colonic cells, as well as their synergistic interaction. Various blends were tested to identify the optimal synergistic effect.
Methods: Biopsies from polyps and healthy colonic tissue were obtained from 22 patients undergoing colonic polypectomies. The toxicity of a variety of cannabinoid extracts and purified cannabinoids at different concentrations was evaluated. The synergistic effect of cannabinoids was calculated based on the cells’ survival.
Isolated cannabinoids illustrated different toxic effects on the viability of cells derived from colorectal polyps. THC-d8 and THC-d9 were the most toxic and exhibited persistent toxicity in all the polyps tested. CBD was more toxic to polypoid cells in comparison to normal colonic cells at a concentration of 15 µM. The combinations of the cannabinoids CBDV, THCV, CBDVA, CBCA, and CBGA exhibited a synergistic inhibitory effect on the viability of cells derived from colon polyps of patients.
Isolated cannabinoid compounds interacted synergistically against colonic polyps, and some also possessed a differential toxic effect on polyp and adjacent colonic tissue, suggesting possible future therapeutic value.”
https://pubmed.ncbi.nlm.nih.gov/36232668/
“To conclude, our study results support the potential cytotoxic effect of cannabinoid extracts on colorectal polyps, as well as their synergistic and differential interactions. Further studies examining this postulation and the ultimate combination of cannabinoids for inhibiting/decreasing the recurrence rate of neoplastic polyps, and for preventing their malignant transformation into adenocarcinoma, are needed.”