“Substantial preclinical evidence demonstrates the antiproliferative, cytotoxic, and antimetastatic properties of plant-derived cannabinoids (phytocannabinoids) such as cannabidiol and tetrahydrocannabinol. The cumulative body of research into the intracellular mechanisms and phenotypic effects of these compounds supports a logical, judicious progression to large-scale phase II/III clinical trials in certain cancer types to truly assess the efficacy of phytocannabinoids as anticancer agents.”
Category Archives: Leukemia
Cannabinoids as anticancer drugs: current status of preclinical research
“Drugs that target the endocannabinoid system are of interest as pharmacological options to combat cancer and to improve the life quality of cancer patients. From this perspective, cannabinoid compounds have been successfully tested as a systemic therapeutic option in a number of preclinical models over the past decades. As a result of these efforts, a large body of data suggests that the anticancer effects of cannabinoids are exerted at multiple levels of tumour progression via different signal transduction mechanisms. Accordingly, there is considerable evidence for cannabinoid-mediated inhibition of tumour cell proliferation, tumour invasion and metastasis, angiogenesis and chemoresistance, as well as induction of apoptosis and autophagy. Further studies showed that cannabinoids could be potential combination partners for established chemotherapeutic agents or other therapeutic interventions in cancer treatment. Research in recent years has yielded several compounds that exert promising effects on tumour cells and tissues in addition to the psychoactive Δ9-tetrahydrocannabinol, such as the non-psychoactive phytocannabinoid cannabidiol and inhibitors of endocannabinoid degradation. This review provides an up-to-date overview of the potential of cannabinoids as inhibitors of tumour growth and spread as demonstrated in preclinical studies.”
Cannabidiol and Other Phytocannabinoids as Cancer Therapeutics
“Preclinical models provided ample evidence that cannabinoids are cytotoxic against cancer cells. Among the best studied phytocannabinoids, cannabidiol (CBD) is most promising for the treatment of cancer as it lacks the psychotomimetic properties of delta-9-tetrahydrocannabinol (THC). In vitro studies and animal experiments point to a concentration- (dose-)dependent anticancer effect. The effectiveness of pure compounds versus extracts is the subject of an ongoing debate. Actual results demonstrate that CBD-rich hemp extracts must be distinguished from THC-rich cannabis preparations. Whereas pure CBD was superior to CBD-rich extracts in most in vitro experiments, the opposite was observed for pure THC and THC-rich extracts, although exceptions were noted. The cytotoxic effects of CBD, THC and extracts seem to depend not only on the nature of cannabinoids and the presence of other phytochemicals but also largely on the nature of cell lines and test conditions. Neither CBD nor THC are universally efficacious in reducing cancer cell viability. The combination of pure cannabinoids may have advantages over single agents, although the optimal ratio seems to depend on the nature of cancer cells; the existence of a ‘one size fits all’ ratio is very unlikely. As cannabinoids interfere with the endocannabinoid system (ECS), a better understanding of the circadian rhythmicity of the ECS, particularly endocannabinoids and receptors, as well as of the rhythmicity of biological processes related to the growth of cancer cells, could enhance the efficacy of a therapy with cannabinoids by optimization of the timing of the administration, as has already been reported for some of the canonical chemotherapeutics. Theoretically, a CBD dose administered at noon could increase the peak of anandamide and therefore the effects triggered by this agent. Despite the abundance of preclinical articles published over the last 2 decades, well-designed controlled clinical trials on CBD in cancer are still missing. The number of observations in cancer patients, paired with the anticancer activity repeatedly reported in preclinical in vitro and in vivo studies warrants serious scientific exploration moving forward.”
Cannabis as a potential compound against various malignancies, legal aspects, advancement by exploiting nanotechnology and clinical trials
“Various preclinical and clinical studies exhibited the potential of cannabis against various diseases, including cancer and related pain. Subsequently, many efforts have been made to establish and develop cannabis-related products and make them available as prescription products. Moreover, FDA has already approved some cannabis-related products, and more advancement in this aspect is still going on. However, the approved product of cannabis is in oral dosage form, which exerts various limitations to achieve maximum therapeutic effects. A considerable translation is on a hike to improve bioavailability, and ultimately, the therapeutic efficacy of cannabis by the employment of nanotechnology. Besides the well-known psychotropic effects of cannabis upon the use at high doses, literature has also shown the importance of cannabis and its constituents in minimising the lethality of cancer in the preclinical models. This review discusses the history of cannabis, its legal aspect, safety profile, the mechanism by which cannabis combats with cancer, and the advancement of clinical therapy by exploiting nanotechnology. A brief discussion related to the role of cannabinoid in various cancers has also been incorporated. Lastly, the information regarding completed and ongoing trials have also been elaborated.”
Chemical characterization of non-psychoactive Cannabis sativa L. extracts, in vitro antiproliferative activity and induction of apoptosis in chronic myelogenous leukaemia cancer cells
“In this study, extracts from non-psychoactive Cannabis sativa L. varieties were characterized by means of ultra high-performance liquid chromatography coupled with high-resolution mass spectrometry (UHPLC-HRMS) and their antiproliferative activity was assessed in vitro. The human chronic myelogenous leukaemia cell line K562 was chosen to investigate the mechanism of cell death. The effect on the cell cycle and cell death was analysed by flow cytometry. Proteins related to apoptosis were studied by western blotting. Mechanical properties of cells were assessed using the Micropipette Aspiration Technique (MAT). The results indicated that the cannabidiol (CBD)-rich extract inhibited cell proliferation of K562 cell line in a dose-dependent manner and induced apoptosis via caspase 3 and 7 activation. A significant decrease in the mitochondrial membrane potential was detected, together with the release of cytochrome c into the cytosol. The main apoptotic markers were not involved in the mechanism of cell death. The extract was also able to modify the mechanical properties of cells. Thus, this hemp extract and its pure component CBD deserve further investigation for a possible application against myeloproliferative diseases, also in association with other anticancer drugs.”
Pros and Cons of the Cannabinoid System in Cancer: Focus on Hematological Malignancies
 “The endocannabinoid system (ECS) is a composite cell-signaling system that allows endogenous cannabinoid ligands to control cell functions through the interaction with cannabinoid receptors. Modifications of the ECS might contribute to the pathogenesis of different diseases, including cancers. However, the use of these compounds as antitumor agents remains debatable.
“The endocannabinoid system (ECS) is a composite cell-signaling system that allows endogenous cannabinoid ligands to control cell functions through the interaction with cannabinoid receptors. Modifications of the ECS might contribute to the pathogenesis of different diseases, including cancers. However, the use of these compounds as antitumor agents remains debatable.
Pre-clinical experimental studies have shown that cannabinoids (CBs) might be effective for the treatment of hematological malignancies, such as leukemia and lymphoma.
Specifically, CBs may activate programmed cell death mechanisms, thus blocking cancer cell growth, and may modulate both autophagy and angiogenesis. Therefore, CBs may have significant anti-tumor effects in hematologic diseases and may synergistically act with chemotherapeutic agents, possibly also reducing chemoresistance.
Moreover, targeting ECS might be considered as a novel approach for the management of graft versus host disease, thus reducing some symptoms such as anorexia, cachexia, fatigue, anxiety, depression, and neuropathic pain. The aim of the present review is to collect the state of the art of CBs effects on hematological tumors, thus focusing on the essential topics that might be useful before moving into the clinical practice.”
https://www.mdpi.com/1420-3049/26/13/3866
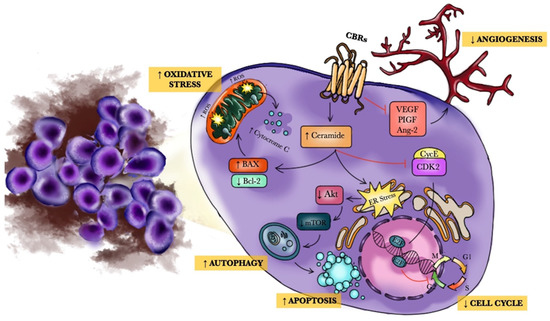
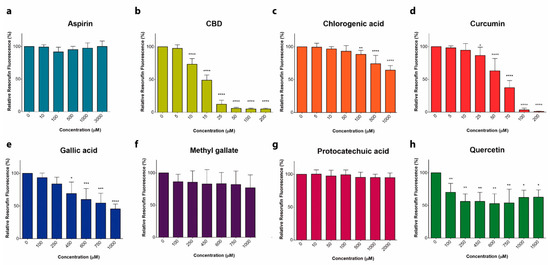
Phenolic Compounds Cannabidiol, Curcumin and Quercetin Cause Mitochondrial Dysfunction and Suppress Acute Lymphoblastic Leukemia Cells
 “Anticancer activity of different phenols is documented, but underlying mechanisms remain elusive. Recently, we have shown that cannabidiol kills the cells of acute lymphoblastic leukemia (ALL) by a direct interaction with mitochondria, with their consequent dysfunction.
“Anticancer activity of different phenols is documented, but underlying mechanisms remain elusive. Recently, we have shown that cannabidiol kills the cells of acute lymphoblastic leukemia (ALL) by a direct interaction with mitochondria, with their consequent dysfunction.
In the present study, cytotoxic effects of several phenolic compounds against human the T-ALL cell line Jurkat were tested by means of resazurin-based metabolic assay. To unravel underlying mechanisms, mitochondrial membrane potential (∆Ψm) and [Ca2+]m measurements were undertaken, and reactive oxygen species generation and cell death were evaluated by flow cytometry.
Three out of eight tested phenolics, cannabidiol, curcumin and quercetin, which displayed a significant cytotoxic effect, also dissipated the ∆Ψm and induced a significant [Ca2+]m increase, whereas inefficient phenols did not.
Dissipation of the ∆Ψm by cannabidiol was prevented by cyclosporine A and reverted by Ru360, inhibitors of the permeation transition pore and mitochondrial Ca2+ uniporter, respectively. Ru360 prevented the phenol-induced [Ca2+]m rise, but neither cyclosporine A nor Ru360 affected the curcumin- and quercetin-induced ∆Ψm depolarization. Ru360 impeded the curcumin- and cannabidiol-induced cell death.
Thus, all three phenols exert their antileukemic activity via mitochondrial Ca2+ overload, whereas curcumin and quercetin suppress the metabolism of leukemic cells by direct mitochondrial uncoupling.”
https://pubmed.ncbi.nlm.nih.gov/33379175/
https://www.mdpi.com/1422-0067/22/1/204
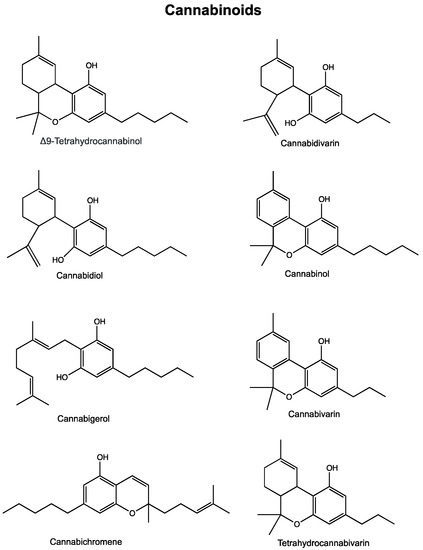
Cannabis and its Constituents for Cancer: History, Biogenesis, Chemistry and Pharmacological Activities
 “Cannabis has long been used for healing and recreation in several regions of the world. Over 400 bioactive constituents, including more than 100 phytocannabinoids, have been isolated from this plant. The non-psychoactive cannabidiol (CBD) and the psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) are the major and widely studied constituents from this plant.
“Cannabis has long been used for healing and recreation in several regions of the world. Over 400 bioactive constituents, including more than 100 phytocannabinoids, have been isolated from this plant. The non-psychoactive cannabidiol (CBD) and the psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) are the major and widely studied constituents from this plant.
Cannabinoids exert their effects through the endocannabinoid system (ECS) that comprises cannabinoid receptors (CB1, CB2), endogenous ligands, and metabolizing enzymes. Several preclinical studies have demonstrated the potential of cannabinoids against leukemia, lymphoma, glioblastoma, and cancers of the breast, colorectum, pancreas, cervix and prostate.
Cannabis and its constituents can modulate multiple cancer related pathways such as PKB, AMPK, CAMKK-β, mTOR, PDHK, HIF-1α, and PPAR-γ. Cannabinoids can block cell growth, progression of cell cycle and induce apoptosis selectively in tumour cells. Cannabinoids can also enhance the efficacy of cancer therapeutics. These compounds have been used for the management of anorexia, queasiness, and pain in cancer patients.
Cannabinoid based products such as dronabinol, nabilone, nabiximols, and epidyolex are now approved for medical use in cancer patients. Cannabinoids are reported to produce a favourable safety profile. However, psychoactive properties and poor bioavailability limit the use of some cannabinoids. The Academic Institutions across the globe are offering training courses on cannabis. How cannabis and its constituents exert anticancer activities is discussed in this article. We also discuss areas that require attention and more extensive research.”
https://pubmed.ncbi.nlm.nih.gov/33246167/
https://www.sciencedirect.com/science/article/abs/pii/S1043661820316108?via%3Dihub
Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis
 “In recent years, and even more since its legalization in several jurisdictions, cannabis and the endocannabinoid system have received an increasing amount of interest related to their potential exploitation in clinical settings. Cannabinoids have been suggested and shown to be effective in the treatment of various conditions. In cancer, the endocannabinoid system is altered in numerous types of tumours and can relate to cancer prognosis and disease outcome. Additionally, cannabinoids display anticancer effects in several models by suppressing the proliferation, migration and/or invasion of cancer cells, as well as tumour angiogenesis. However, the therapeutic use of cannabinoids is currently limited to the treatment of symptoms and pain associated with chemotherapy, while their potential use as cytotoxic drugs in chemotherapy still requires validation in patients. Along with cannabinoids, cannabis contains several other compounds that have also been shown to exert anti-tumorigenic actions. The potential anti-cancer effects of cannabinoids, terpenes and flavonoids, present in cannabis, are explored in this literature review.”
“In recent years, and even more since its legalization in several jurisdictions, cannabis and the endocannabinoid system have received an increasing amount of interest related to their potential exploitation in clinical settings. Cannabinoids have been suggested and shown to be effective in the treatment of various conditions. In cancer, the endocannabinoid system is altered in numerous types of tumours and can relate to cancer prognosis and disease outcome. Additionally, cannabinoids display anticancer effects in several models by suppressing the proliferation, migration and/or invasion of cancer cells, as well as tumour angiogenesis. However, the therapeutic use of cannabinoids is currently limited to the treatment of symptoms and pain associated with chemotherapy, while their potential use as cytotoxic drugs in chemotherapy still requires validation in patients. Along with cannabinoids, cannabis contains several other compounds that have also been shown to exert anti-tumorigenic actions. The potential anti-cancer effects of cannabinoids, terpenes and flavonoids, present in cannabis, are explored in this literature review.”
https://pubmed.ncbi.nlm.nih.gov/32708138/
https://www.mdpi.com/2072-6694/12/7/1985
Cannabinoid CP55940 Selectively Induces Apoptosis in Jurkat Cells and in Ex Vivo T-cell Acute Lymphoblastic Leukemia Through H 2 O 2 Signaling Mechanism
 ‘T-cell acute lymphoblastic leukemia (T-ALL) is a highly heterogeneous malignant hematological disorder arising from T-cell progenitors.
‘T-cell acute lymphoblastic leukemia (T-ALL) is a highly heterogeneous malignant hematological disorder arising from T-cell progenitors.
This study was aimed to evaluate the cytotoxic effect of CP55940 on human peripheral blood lymphocytes (PBL) and on T-ALL cells (Jurkat).
In conclusion, CP55940 selectively induces apoptosis in Jurkat cells through a H2O2-mediated signaling pathway.
Our findings support the use of cannabinoids as a potential treatment for T-ALL cells.”
https://pubmed.ncbi.nlm.nih.gov/32540572/
https://www.sciencedirect.com/science/article/abs/pii/S0145212620300941?via%3Dihub
“CP 55,940 is a synthetic cannabinoid which mimics the effects of naturally occurring THC (one of the psychoactive compounds found in cannabis)” https://en.wikipedia.org/wiki/CP_55,940
