 “In recent years, evidence has accumulated that cannabinoids-especially the non-psychoactive compound, cannabidiol (CBD)-possess promising medical and pharmacological activities that might qualify them as potential anti-tumor drugs. This review is based on multiple studies summarizing different mechanisms for how CBD can target tumor cells including cannabinoid receptors or other constituents of the endocannabinoid system, and their complex activation of biological systems that results in the inhibition of tumor growth. CBD also participates in anti-inflammatory activities which are related to tumor progression, as demonstrated in preclinical models. Although the numbers of clinical trials and tested tumor entities are limited, there is clear evidence that CBD has anti-tumor efficacy and is well tolerated in human cancer patients. In summary, it appears that CBD has potential as a neoadjuvant and/or adjuvant drug in therapy for cancer.”
“In recent years, evidence has accumulated that cannabinoids-especially the non-psychoactive compound, cannabidiol (CBD)-possess promising medical and pharmacological activities that might qualify them as potential anti-tumor drugs. This review is based on multiple studies summarizing different mechanisms for how CBD can target tumor cells including cannabinoid receptors or other constituents of the endocannabinoid system, and their complex activation of biological systems that results in the inhibition of tumor growth. CBD also participates in anti-inflammatory activities which are related to tumor progression, as demonstrated in preclinical models. Although the numbers of clinical trials and tested tumor entities are limited, there is clear evidence that CBD has anti-tumor efficacy and is well tolerated in human cancer patients. In summary, it appears that CBD has potential as a neoadjuvant and/or adjuvant drug in therapy for cancer.”
https://pubmed.ncbi.nlm.nih.gov/33921049/
“It has been shown that CBD, either alone or in combination with other therapies, has the potential to act as a novel anti-tumor, anti-inflammatory and anti-pain drug in preclinical studies and first clinical trials. A few clinical trials have now demonstrated beneficial pharmacokinetic and pharmacodynamic characteristics of the drug, and some anti-tumor activities at well-tolerated doses. Therefore, it can be assumed that CBD might be considered a potential candidate for neoadjuvant and/or adjuvant interventions in oncology.”

 “Cannabinoids are a group of chemicals that bind to receptors in the human body and, in turn, modulate the endocannabinoid system (ECS). They can be endogenously produced, synthetic, or derived from the plant Cannabis sativa L.
“Cannabinoids are a group of chemicals that bind to receptors in the human body and, in turn, modulate the endocannabinoid system (ECS). They can be endogenously produced, synthetic, or derived from the plant Cannabis sativa L. “Background: Medical cannabis use is increasing rapidly in the past several years, with older adults being the fastest growing group. Nevertheless, the evidence for cardiovascular safety of cannabis use is scarce. The aim of this study was to assess the effect of cannabis on blood pressure, heart rate, and metabolic parameters in older adults with hypertension.
“Background: Medical cannabis use is increasing rapidly in the past several years, with older adults being the fastest growing group. Nevertheless, the evidence for cardiovascular safety of cannabis use is scarce. The aim of this study was to assess the effect of cannabis on blood pressure, heart rate, and metabolic parameters in older adults with hypertension. “Recently, there has been a growing interest in the medical applications of Cannabis plants. They owe their unique properties to a group of secondary metabolites known as phytocannabinoids, which are specific for this genus. Phytocannabinoids, and cannabinoids generally, can interact with cannabinoid receptors being part of the endocannabinoid system present in animals. Over the years a growing body of scientific evidence has been gathered, suggesting that these compounds have therapeutic potential.
“Recently, there has been a growing interest in the medical applications of Cannabis plants. They owe their unique properties to a group of secondary metabolites known as phytocannabinoids, which are specific for this genus. Phytocannabinoids, and cannabinoids generally, can interact with cannabinoid receptors being part of the endocannabinoid system present in animals. Over the years a growing body of scientific evidence has been gathered, suggesting that these compounds have therapeutic potential.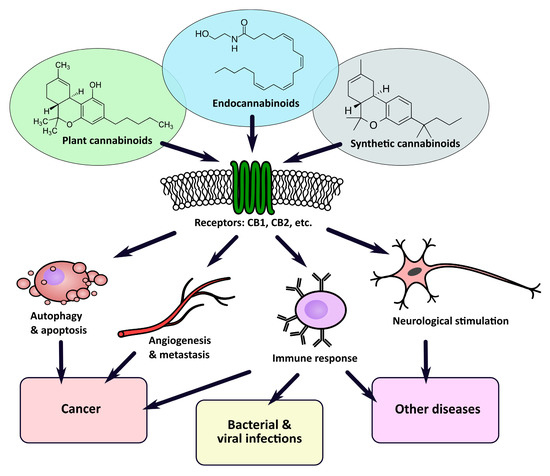
 “In the last decade the use of medical cannabis (MC) for palliative cancer treatment has risen. However, the choice between products is arbitrary and most patients are using Tetrahydrocannabinol (THC)-dominant cannabis products.
“In the last decade the use of medical cannabis (MC) for palliative cancer treatment has risen. However, the choice between products is arbitrary and most patients are using Tetrahydrocannabinol (THC)-dominant cannabis products. “There is not a single pharmacological agent with demonstrated therapeutic efficacy for traumatic brain injury (TBI). With recent legalization efforts and the growing popularity of medical cannabis, patients with TBI will inevitably consider medical cannabis as a treatment option.
“There is not a single pharmacological agent with demonstrated therapeutic efficacy for traumatic brain injury (TBI). With recent legalization efforts and the growing popularity of medical cannabis, patients with TBI will inevitably consider medical cannabis as a treatment option. “Opioid misuse and overuse has contributed to a widespread overdose crisis and many patients and physicians are considering medical cannabis to support opioid tapering and chronic pain control. Using a five-step modified Delphi process, we aimed to develop consensus-based recommendations on: 1) when and how to safely initiate and titrate cannabinoids in the presence of opioids, 2) when and how to safely taper opioids in the presence of cannabinoids, and 3) how to monitor patients and evaluate outcomes when treating with opioids and cannabinoids.
“Opioid misuse and overuse has contributed to a widespread overdose crisis and many patients and physicians are considering medical cannabis to support opioid tapering and chronic pain control. Using a five-step modified Delphi process, we aimed to develop consensus-based recommendations on: 1) when and how to safely initiate and titrate cannabinoids in the presence of opioids, 2) when and how to safely taper opioids in the presence of cannabinoids, and 3) how to monitor patients and evaluate outcomes when treating with opioids and cannabinoids. “Research within a gynecologic oncology population has lagged behind the uptake in use of medical cannabis for symptom control. This study seeks to evaluate patient experience with prescribed medical cannabis obtained through licensed dispensaries in women with gynecologic malignancies.
“Research within a gynecologic oncology population has lagged behind the uptake in use of medical cannabis for symptom control. This study seeks to evaluate patient experience with prescribed medical cannabis obtained through licensed dispensaries in women with gynecologic malignancies. “Little is known about the patients’ view on treatment with medical cannabis (MC) for Parkinson’s disease (PD).
“Little is known about the patients’ view on treatment with medical cannabis (MC) for Parkinson’s disease (PD). “With the current COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), there is an urgent need for new therapies and prevention strategies that can help curtail disease spread and reduce mortality.
“With the current COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), there is an urgent need for new therapies and prevention strategies that can help curtail disease spread and reduce mortality.