“Cannabinoids (CBs), analgesic drugs used for thousands of years, were first found in Cannabis sativa, and the multiple CBs used medicinally, such as tetrahydrocannabinol (THC), cannabidiol (CBD) and dozens more, have complex structures. In addition to their production by plants, CBs are naturally present in the nerves and immune systems of humans and animals.
“Cannabinoids (CBs), analgesic drugs used for thousands of years, were first found in Cannabis sativa, and the multiple CBs used medicinally, such as tetrahydrocannabinol (THC), cannabidiol (CBD) and dozens more, have complex structures. In addition to their production by plants, CBs are naturally present in the nerves and immune systems of humans and animals.
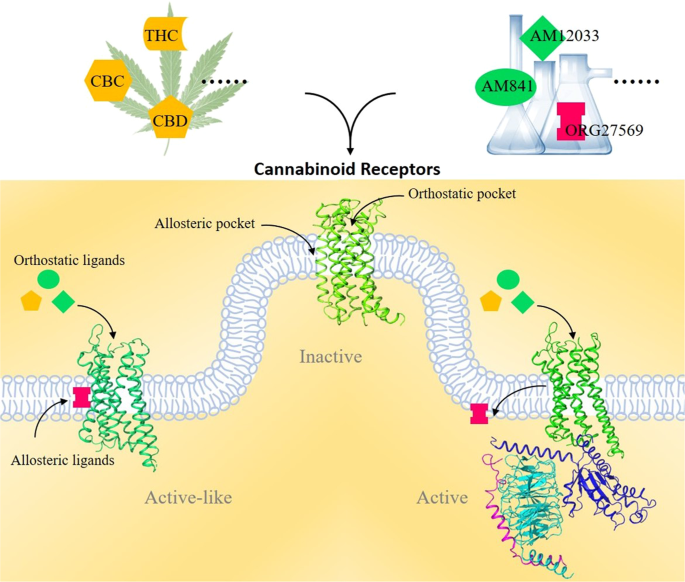
Both exogenous and endogenous CBs carry out a variety of physiological functions by engaging with two CB receptors, the CB1 and CB2 receptors, in the human endocannabinoid system (ECS). Both CB1 and CB2 are G protein-coupled receptors that share a 7-transmembrane (7TM) topology. CB1, known as the central CB receptor, is mainly distributed in the brain, spinal cord, and peripheral nervous system. CB1 activation in the human body typically promotes the release of neurotransmitters, controls pain and memory learning, and regulates metabolism and the cardiovascular system.
Clinically, CB1 is a direct drug target for drug addiction, neurodegenerative diseases, pain, epilepsy, and obesity. Unlike the exclusive expression of CB1 in the nervous system, CB2 is mainly distributed in peripheral immune cells. Selective CB2 agonists would have therapeutic potential in the treatment of inflammation and pain and avoid side effects caused by currently used clinical drugs.
Although significant progress has been made in developing agonists toward CB receptors, efficient clinical drugs targeting CB receptors remain lacking due to their complex signaling mechanisms. The recent structural elucidation of CB receptors has greatly aided our understanding of the activation and signal transduction mechanisms of CB receptors.
Recent structural characterizations of CB receptors will greatly facilitate the design of new ligands to modulate the selective functions of CB receptors. Notably, the CBD was approved by the Food and Drug Administration (FDA) in 2018 to treat epilepsy. We now look forward to more drugs targeting these two CB receptors for clinical usage in the near future.”