“HIV-associated neurocognitive disorders (HAND) persist despite the advent of antiretroviral therapy (ART), suggesting underlying systemic and central nervous system (CNS) inflammatory mechanisms.
“HIV-associated neurocognitive disorders (HAND) persist despite the advent of antiretroviral therapy (ART), suggesting underlying systemic and central nervous system (CNS) inflammatory mechanisms.
The endogenous cannabinoid receptors 1 and 2 (CB1 and CB2) modulate inflammatory gene expression and play an important role in maintaining neuronal homeostasis. Cannabis use is disproportionately high among people with HIV (PWH) and may provide a neuroprotective effect for those on ART due to its anti-inflammatory properties. However, expression profiles of CB1 and CB2 in the brains of PWH on ART with HAND have not been reported.
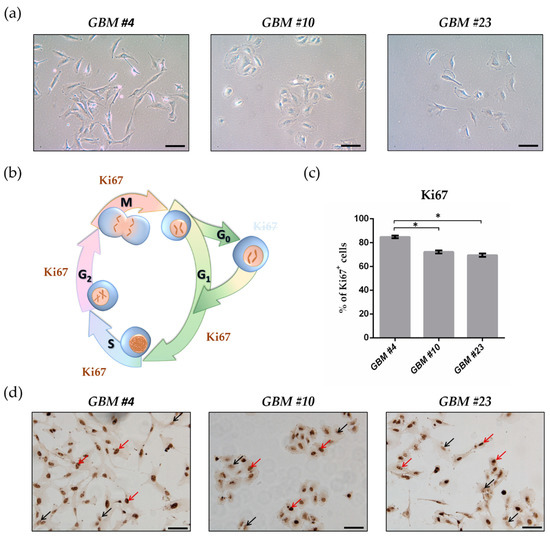
In this study, biochemical and immunohistochemical analyses were performed to determine CB1 and CB2 expression in the brain specimens of HAND donors.
Immunoblot revealed that CB1 and CB2 were differentially expressed in the frontal cortices of HAND brains compared to neurocognitively unimpaired (NUI) brains of PWH. CB1 expression levels negatively correlated with memory and information processing speed. CB1 was primarily localized to neuronal soma in HAND brains versus a more punctate distribution of neuronal processes in NUI brains. CB1 expression was increased in cells with glial morphology and showed increased colocalization with an astroglial marker.
These results suggest that targeting the endocannabinoid system may be a potential therapeutic strategy for HAND.”

 “The global incidence of respiratory diseases and complications is increasing. Therefore, new methods of treatment, as well as prevention, need to be investigated.
“The global incidence of respiratory diseases and complications is increasing. Therefore, new methods of treatment, as well as prevention, need to be investigated.  “Cannabinoids, active components of the plant
“Cannabinoids, active components of the plant  “Colic is a common digestive disorder in horses and one of the most urgent problems in equine medicine. A growing body of literature has indicated that the activation of cannabinoid receptors could exert beneficial effects on gastrointestinal inflammation and visceral hypersensitivity.
“Colic is a common digestive disorder in horses and one of the most urgent problems in equine medicine. A growing body of literature has indicated that the activation of cannabinoid receptors could exert beneficial effects on gastrointestinal inflammation and visceral hypersensitivity.  “Cannabis sativa
“Cannabis sativa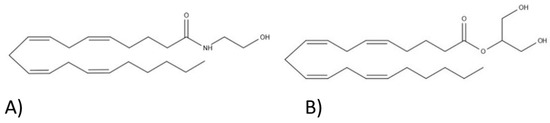
 “In humans, various sites like cannabinoid receptors (CBR) having a binding affinity with cannabinoids are distributed on the surface of different cell types, where endocannabinoids (ECs) and derivatives of fatty acid can bind. The binding of these substance(s) triggers the activation of specific receptors required for various physiological functions, including pain sensation, memory, and appetite.
“In humans, various sites like cannabinoid receptors (CBR) having a binding affinity with cannabinoids are distributed on the surface of different cell types, where endocannabinoids (ECs) and derivatives of fatty acid can bind. The binding of these substance(s) triggers the activation of specific receptors required for various physiological functions, including pain sensation, memory, and appetite. 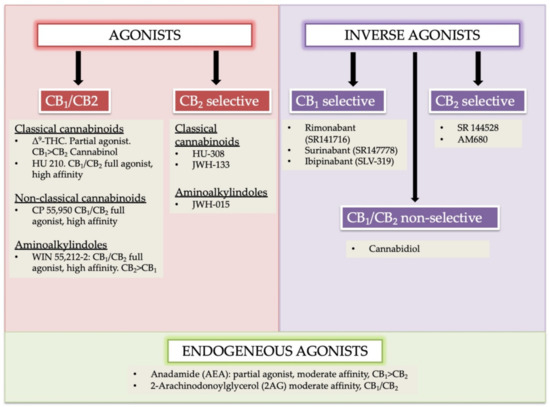
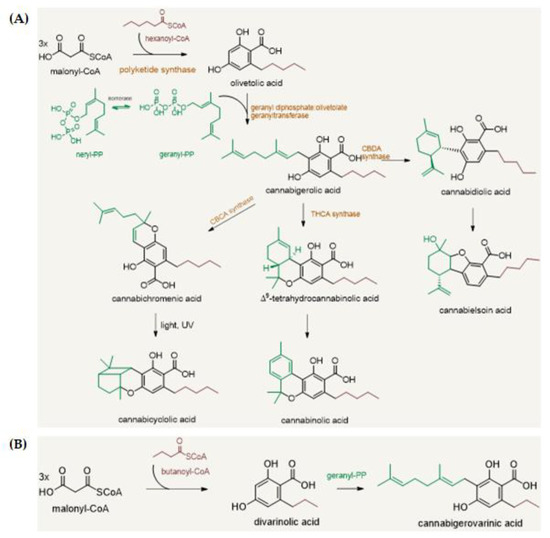
 “The therapeutic potential of
“The therapeutic potential of 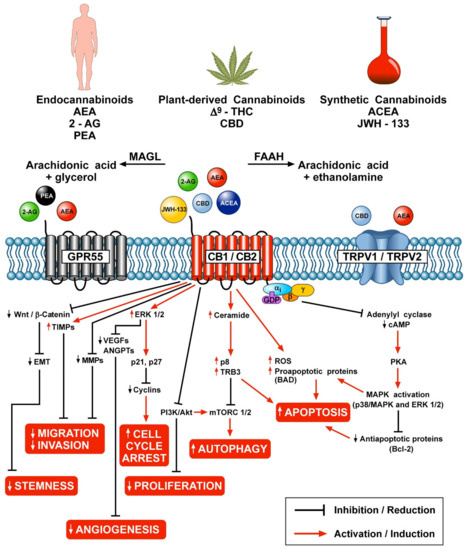
 “
“