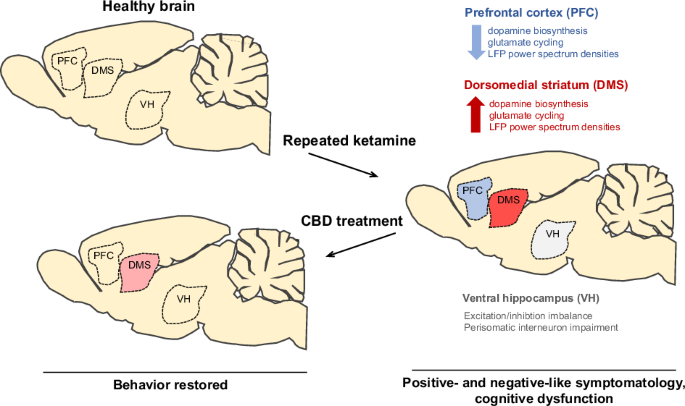
“Cannabidiol (CBD) has shown potential for treating schizophrenia (SCZ) by targeting its positive and negative cognitive symptoms. In this study, we investigated if CBD could reverse the memory impairment observed after chronic administration of the NMDA receptor antagonist MK-801.
Chronic treatment with MK-801 (0.5 mg/kg i.p., twice a day, for 14 days) resulted in short- and long-term memory deficits and decreased relative power of γ oscillations in freely moving animals. CBD administration (30 mg/kg i.p. daily for seven days after the MK-801 treatment period) reversed these changes. The cognitive effects of CBD were prevented by blocking 5-HT1A but not CB2 receptors.
At the cellular level, the depletion of parvalbumin-positive neurons and their associated perineuronal nets in the prelimbic medial prefrontal cortex (mPFC) and ventral hippocampus (vHip) induced by MK-801 was reversed by CBD. This neuroprotective effect was mediated by 5-HT1A and CB2 receptors in the vHip but was independent of these receptors in the mPFC. Additionally, CBD reversed MK-801-induced microglial activation in both mPFC and vHip, again through 5-HT1A and CB2 receptors.
These findings suggest that CBD modulates multiple pathways affected in SCZ-like conditions, offering a promising therapeutic avenue for SCZ treatment.”
https://pubmed.ncbi.nlm.nih.gov/40484109/
https://www.sciencedirect.com/science/article/abs/pii/S0006899325003336?via%3Dihub