
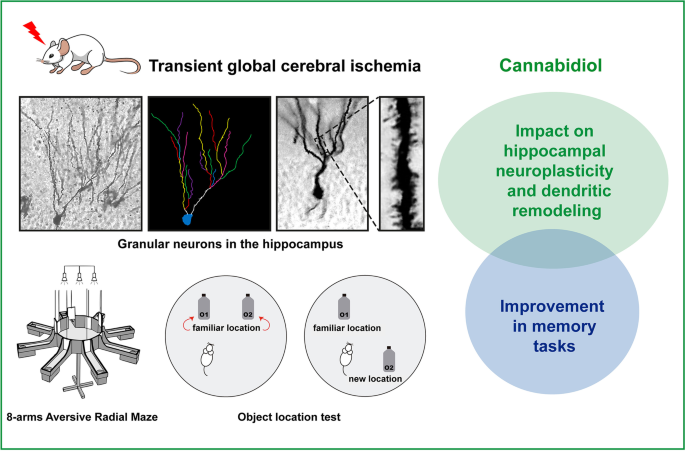
“The absence of blood flow in cerebral ischemic conditions triggers a multitude of intricate pathophysiological mechanisms, including excitotoxicity, oxidative stress, neuroinflammation, disruption of the blood-brain barrier and white matter disarrangement. Despite numerous experimental studies conducted in preclinical settings, existing treatments for cerebral ischemia (CI), such as mechanical and pharmacological therapies, remain constrained and often entail significant side effects. Therefore, there is an imperative to explore innovative strategies for addressing CI outcomes.
Cannabidiol (CBD), the most abundant non-psychotomimetic compound derived from Cannabis sativa, is a pleiotropic substance that interacts with diverse molecular targets and has the potential to influence various pathophysiological processes, thereby contributing to enhanced outcomes in CI. This chapter provides a comprehensive overview of the primary effects of CBD in in vitro and diverse animal models of CI and delves into some of its plausible mechanisms of neuroprotection.”
https://pubmed.ncbi.nlm.nih.gov/39029992/
“CBD emerges as a promising therapeutic agent for the treatment and management of cerebral ischemic conditions. The intricate pathogenesis of CI, coupled with CBD’s multitarget effects, underscores its potential as a neuroprotective agent. “
https://www.sciencedirect.com/science/article/abs/pii/S0074774224000643?via%3Dihub



 “The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems.
“The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems. 
 “Cannabidiol (CBD), a non-euphorigenic compound derived from
“Cannabidiol (CBD), a non-euphorigenic compound derived from 

