“Cannabis sativa L. is an annual herbaceous dioecious plant which was first cultivated by agricultural human societies in Asia. Over the period of time, various parts of the plant like leaf, flower, and seed were used for recreational as well as therapeutic purposes. The main chemical components of Cannabis sativa are termed as cannabinoids, among them the key psychoactive constituent is Δ-9-tetrahydrocannabinol and cannabidiol (CBD) as active nonpsychotic constituent. Upon doing extensive literature review, it was found that cannabis has been widely studied for a number of disorders. Very recently, a pure CBD formulation, named Epidiolex, got a green flag from both United States Food and Drug Administration and Drug Enforcement Administration for 2 rare types of epilepsies. This laid a milestone in medical cannabis research.
This review intends to give a basic and extensive assessment, from past till present, of the ethnological, plant, chemical, pharmacological, and legal aspects of C. sativa. Further, this review contemplates the evidence the studies obtained of cannabis components on Alzheimer’s, Parkinson’s, amyotrophic lateral sclerosis, multiple sclerosis, emesis, epilepsy, chronic pain, and cancer as a cytotoxic agent as well as a palliative therapy. The assessment in this study was done by reviewing in extensive details from studies on historical importance, ethnopharmacological aspects, and legal grounds of C. sativa from extensive literature available on the scientific databases, with a vision for elevating further pharmaceutical research to investigate its total potential as a therapeutic agent.”
https://pubmed.ncbi.nlm.nih.gov/34676349/
“This study has analyzed and reviewed the historical, botanical, chemical, ethnopharmacological, and legal aspects of C. sativa from the first human use to the present medical applications with an analysis of its multiple therapeutic applications for various diseased conditions in the contemporary scientific context. There is an abundance of support for its several medicative uses as well as a possible benefit in various diseased conditions. Extensive pharmacological examination is still needed to better understand the clinical significance and uses of active cannabinoids in the treatment and prevention of chronic diseases. Also, cannabis can be chemically standardized and under prescription can be used. With the majority of the United States currently legalizing medicinal cannabis and/or restricted CBD-only use, physicians need to be educated on the history and correct clinical use of cannabis, as a result of which patients can know more and more about possible treatment utilizing cannabis. Medical cannabis has shown to have clinical efficacy in our past, and in present, data show its therapeutic effects. Extensive research in the field of cannabis can be very fruitful for the medicine world.”

 “The concept of neurons as irreplaceable cells does not hold true today. Experiments and evidence of neurogenesis, also, in the adult brain give hope that some compounds or drugs can enhance this process, helping to reverse the outcomes of diseases or traumas that once were thought to be everlasting.
“The concept of neurons as irreplaceable cells does not hold true today. Experiments and evidence of neurogenesis, also, in the adult brain give hope that some compounds or drugs can enhance this process, helping to reverse the outcomes of diseases or traumas that once were thought to be everlasting.  “Pain prevalence among adults in the United States has increased 25% over the past two decades, resulting in high health-care costs and impacts to patient quality of life. In the last 30 years, our understanding of pain circuits and (intra)cellular mechanisms has grown exponentially, but this understanding has not yet resulted in improved therapies. Options for pain management are limited. Many analgesics have poor efficacy and are accompanied by severe side effects such as addiction, resulting in a devastating opioid abuse and overdose epidemic. These problems have encouraged scientists to identify novel molecular targets and develop alternative pain therapeutics.
“Pain prevalence among adults in the United States has increased 25% over the past two decades, resulting in high health-care costs and impacts to patient quality of life. In the last 30 years, our understanding of pain circuits and (intra)cellular mechanisms has grown exponentially, but this understanding has not yet resulted in improved therapies. Options for pain management are limited. Many analgesics have poor efficacy and are accompanied by severe side effects such as addiction, resulting in a devastating opioid abuse and overdose epidemic. These problems have encouraged scientists to identify novel molecular targets and develop alternative pain therapeutics. “Aging is a complex phenomenon associated with a wide spectrum of physical and physiological changes affecting every part of all metazoans, if they escape death prior to reaching maturity. Critical to survival, the immune system evolved as the principal component of response to injury and defense against pathogen invasions. Because how significantly immune system affects and is affected by aging, several neologisms now appear to encapsulate these reciprocal relationships, such as Immunosenescence. The central part of Immunosenescence is Inflammaging -a sustained, low-grade, sterile inflammation occurring after reaching reproductive prime. Once initiated, the impact of Inflammaging and its adverse effects determine the direction and magnitudes of further Inflammaging. In this article, we review the nature of this vicious cycle, we will propose that phytocannabinoids as immune regulators may possess the potential as effective adjunctive therapies to slow and, in certain cases, reverse the pathologic senescence to permit a more healthy aging.”
“Aging is a complex phenomenon associated with a wide spectrum of physical and physiological changes affecting every part of all metazoans, if they escape death prior to reaching maturity. Critical to survival, the immune system evolved as the principal component of response to injury and defense against pathogen invasions. Because how significantly immune system affects and is affected by aging, several neologisms now appear to encapsulate these reciprocal relationships, such as Immunosenescence. The central part of Immunosenescence is Inflammaging -a sustained, low-grade, sterile inflammation occurring after reaching reproductive prime. Once initiated, the impact of Inflammaging and its adverse effects determine the direction and magnitudes of further Inflammaging. In this article, we review the nature of this vicious cycle, we will propose that phytocannabinoids as immune regulators may possess the potential as effective adjunctive therapies to slow and, in certain cases, reverse the pathologic senescence to permit a more healthy aging.” “Purpose:
“Purpose: 
 “Chronic hepatitis B virus (HBV) infection may evolve into cirrhosis and hepatocellular carcinoma, and this progression may be accelerated by specific risk factors, including overweight and obesity. Although evidence for a protective effect of cannabis use on elevated body weight has been found for other populations, no data are available for HBV-infected patients.
“Chronic hepatitis B virus (HBV) infection may evolve into cirrhosis and hepatocellular carcinoma, and this progression may be accelerated by specific risk factors, including overweight and obesity. Although evidence for a protective effect of cannabis use on elevated body weight has been found for other populations, no data are available for HBV-infected patients. 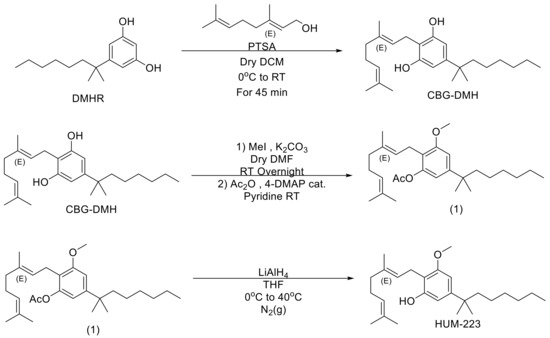
 “The global incidence of respiratory diseases and complications is increasing. Therefore, new methods of treatment, as well as prevention, need to be investigated.
“The global incidence of respiratory diseases and complications is increasing. Therefore, new methods of treatment, as well as prevention, need to be investigated.  “THC, CBD, and CBN were reported as promising candidates against SARS-CoV2 infection, but the mechanism of action of these three cannabinoids is not understood.
“THC, CBD, and CBN were reported as promising candidates against SARS-CoV2 infection, but the mechanism of action of these three cannabinoids is not understood.  “Conventional lung cancer treatments include surgery, chemotherapy and radiotherapy; however, these treatments are often poorly tolerated by patients. Cannabinoids have been studied for use as a primary cancer treatment. Cannabinoids, which are chemically similar to our own body’s endocannabinoids, can interact with signalling pathways to control the fate of cells, including cancer cells. We present a patient who declined conventional lung cancer treatment. Without the knowledge of her clinicians, she chose to self-administer ‘cannabidiol (CBD) oil’ orally 2-3 times daily. Serial imaging shows that her cancer reduced in size progressively from 41 mm to 10 mm over a period of 2.5 years. Previous studies have failed to agree on the usefulness of cannabinoids as a cancer treatment. This case appears to demonstrate a possible benefit of ‘CBD oil’ intake that may have resulted in the observed tumour regression. The use of cannabinoids as a potential cancer treatment justifies further research.”
“Conventional lung cancer treatments include surgery, chemotherapy and radiotherapy; however, these treatments are often poorly tolerated by patients. Cannabinoids have been studied for use as a primary cancer treatment. Cannabinoids, which are chemically similar to our own body’s endocannabinoids, can interact with signalling pathways to control the fate of cells, including cancer cells. We present a patient who declined conventional lung cancer treatment. Without the knowledge of her clinicians, she chose to self-administer ‘cannabidiol (CBD) oil’ orally 2-3 times daily. Serial imaging shows that her cancer reduced in size progressively from 41 mm to 10 mm over a period of 2.5 years. Previous studies have failed to agree on the usefulness of cannabinoids as a cancer treatment. This case appears to demonstrate a possible benefit of ‘CBD oil’ intake that may have resulted in the observed tumour regression. The use of cannabinoids as a potential cancer treatment justifies further research.”