 “Evidence on the use and efficacy of medical cannabis for children is limited. We examined clinical and epidemiological characteristics of medical cannabis treatment and caregiver-reported effects in children and adolescents in Switzerland.
“Evidence on the use and efficacy of medical cannabis for children is limited. We examined clinical and epidemiological characteristics of medical cannabis treatment and caregiver-reported effects in children and adolescents in Switzerland.
We collected clinical data from children and adolescents (< 18 years) who received Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), or a combination of the two between 2008 and 2019 in Switzerland. Out of 205 contacted families, 90 agreed to participate. The median age at the first prescription was 11.5 years (interquartile range (IQR) 6-16), and 32 patients were female (36%). Fifty-one (57%) patients received CBD only and 39 (43%) THC. Patients were more likely to receive THC therapy if one of the following symptoms or signs were present: spasticity, pain, lack of weight gain, vomiting, or nausea, whereas seizures were the dominant indication for CBD therapy.
Improvements were reported in 59 (66%) study participants.
The largest treatment effects were reported for pain, spasticity, and frequency of seizures in participants treated with THC, and for those treated with pure CBD, the frequency of seizures. However, 43% of caregivers reported treatment interruptions, mainly because of lack of improvement (56%), side effects (46%), the need for a gastric tube (44%), and cost considerations (23%).
Conclusions: The effects of medical cannabis in children and adolescents with chronic conditions are unknown except for rare seizure disorders, but the caregiver-reported data analysed here may justify trials of medical cannabis with standardized concentrations of THC or CBD to assess its efficacy in the young.
What is Known: • The use of medical cannabis (THC and CBD) to treat a variety of diseases among children and adolescents is increasing. • In contrast to adults, there is no evidence to support the use of medical cannabis to treat chronic pain and spasticity in children, but substantial evidence to support the use of CBD in children with rare seizure disorders.
What is New: • This study provides important insights into prescription practices, dosages, and treatment outcomes in children and adolescents using medical cannabis data from a real-life setting.
• The effects of medical cannabis in children and adolescents with chronic conditions shown in our study support trials of medical cannabis for chronic conditions.”
https://pubmed.ncbi.nlm.nih.gov/34309706/
“For two thirds of participants treated with standardized THC or CBD preparations, the caregiver reported an improvement in their condition and well-being. Medical cannabis could be a promising and useful therapy for children and adolescents with neurological conditions.”
 “Aging is a complex phenomenon associated with a wide spectrum of physical and physiological changes affecting every part of all metazoans, if they escape death prior to reaching maturity. Critical to survival, the immune system evolved as the principal component of response to injury and defense against pathogen invasions. Because how significantly immune system affects and is affected by aging, several neologisms now appear to encapsulate these reciprocal relationships, such as Immunosenescence. The central part of Immunosenescence is Inflammaging -a sustained, low-grade, sterile inflammation occurring after reaching reproductive prime. Once initiated, the impact of Inflammaging and its adverse effects determine the direction and magnitudes of further Inflammaging. In this article, we review the nature of this vicious cycle, we will propose that phytocannabinoids as immune regulators may possess the potential as effective adjunctive therapies to slow and, in certain cases, reverse the pathologic senescence to permit a more healthy aging.”
“Aging is a complex phenomenon associated with a wide spectrum of physical and physiological changes affecting every part of all metazoans, if they escape death prior to reaching maturity. Critical to survival, the immune system evolved as the principal component of response to injury and defense against pathogen invasions. Because how significantly immune system affects and is affected by aging, several neologisms now appear to encapsulate these reciprocal relationships, such as Immunosenescence. The central part of Immunosenescence is Inflammaging -a sustained, low-grade, sterile inflammation occurring after reaching reproductive prime. Once initiated, the impact of Inflammaging and its adverse effects determine the direction and magnitudes of further Inflammaging. In this article, we review the nature of this vicious cycle, we will propose that phytocannabinoids as immune regulators may possess the potential as effective adjunctive therapies to slow and, in certain cases, reverse the pathologic senescence to permit a more healthy aging.”
 “Cannabidiol (CBD), a primary bioactive phytocannabinoid extracted from hemp, is reported to possess potent anti-tumorigenic activity in multiple cancers.
“Cannabidiol (CBD), a primary bioactive phytocannabinoid extracted from hemp, is reported to possess potent anti-tumorigenic activity in multiple cancers.  “Cannabidiol (CBD), a phytochemical derived from
“Cannabidiol (CBD), a phytochemical derived from  “Background:
“Background:  “Introduction:
“Introduction: “Evidence on the use and efficacy of medical cannabis for children is limited. We examined clinical and epidemiological characteristics of medical cannabis treatment and caregiver-reported effects in children and adolescents in Switzerland.
“Evidence on the use and efficacy of medical cannabis for children is limited. We examined clinical and epidemiological characteristics of medical cannabis treatment and caregiver-reported effects in children and adolescents in Switzerland.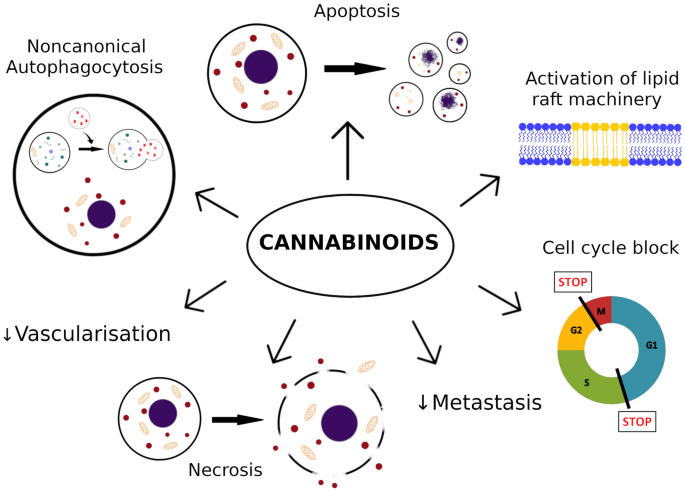
 “Cannabinoids, including delta-8- and delta-9-tetrahydrocannabinol (THC) have a palliative care impact and may therefore be beneficial against cancer.
“Cannabinoids, including delta-8- and delta-9-tetrahydrocannabinol (THC) have a palliative care impact and may therefore be beneficial against cancer. 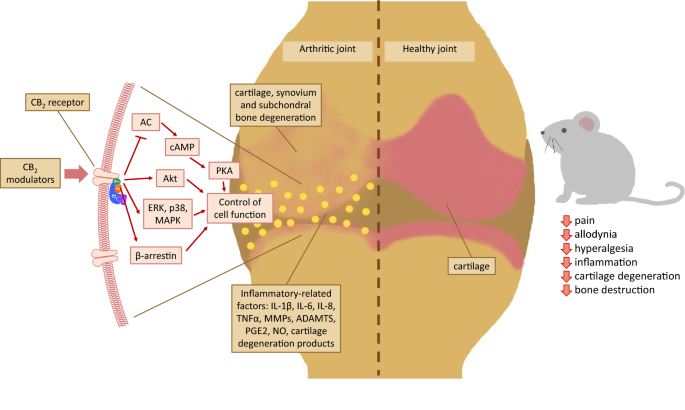 “Over the last several decades, the percentage of patients suffering from different forms of arthritis has increased due to the ageing population and the increasing risk of civilization diseases, e.g. obesity, which contributes to arthritis development. Osteoarthritis and rheumatoid arthritis are estimated to affect 50-60% of people over 65 years old and cause serious health and economic problems. Currently, therapeutic strategies are limited and focus mainly on pain attenuation and maintaining joint functionality. First-line therapies are nonsteroidal anti-inflammatory drugs; in more advanced stages, stronger analgesics, such as opioids, are required, and in the most severe cases, joint arthroplasty is the only option to ensure joint mobility.
“Over the last several decades, the percentage of patients suffering from different forms of arthritis has increased due to the ageing population and the increasing risk of civilization diseases, e.g. obesity, which contributes to arthritis development. Osteoarthritis and rheumatoid arthritis are estimated to affect 50-60% of people over 65 years old and cause serious health and economic problems. Currently, therapeutic strategies are limited and focus mainly on pain attenuation and maintaining joint functionality. First-line therapies are nonsteroidal anti-inflammatory drugs; in more advanced stages, stronger analgesics, such as opioids, are required, and in the most severe cases, joint arthroplasty is the only option to ensure joint mobility.  “Foodborne protein hydrolysates exhibit biological activity that may be therapeutic in a number of human disease settings. Hemp peptides (HP) generated by controlled hydrolysis of hemp proteins have a number of health benefits and are of pharmaceutical value. In the present study, we produce small molecular weight HP from hemp seed and investigate its anticancer properties in Hep3B human liver cancer cells. We demonstrate that HP treatment increased apoptosis, reduced cell viability, and reduced cell migration in Hep3B human liver cancer cells without affecting the normal liver cell line L02. We correlate these phenotypes with increased cellular ROS levels, upregulation of cleaved caspase 3 and Bad, and downregulation of antiapoptotic Bcl-2. HP treatment led to increased Akt and GSK-3β phosphorylation, with subsequent downregulation of β-catenin, suggesting β-catenin signaling modulation as a critical mechanism by which HP exhibits anticancer properties. Our findings suggest HP are of potential therapeutic interest for liver cancer treatment.”
“Foodborne protein hydrolysates exhibit biological activity that may be therapeutic in a number of human disease settings. Hemp peptides (HP) generated by controlled hydrolysis of hemp proteins have a number of health benefits and are of pharmaceutical value. In the present study, we produce small molecular weight HP from hemp seed and investigate its anticancer properties in Hep3B human liver cancer cells. We demonstrate that HP treatment increased apoptosis, reduced cell viability, and reduced cell migration in Hep3B human liver cancer cells without affecting the normal liver cell line L02. We correlate these phenotypes with increased cellular ROS levels, upregulation of cleaved caspase 3 and Bad, and downregulation of antiapoptotic Bcl-2. HP treatment led to increased Akt and GSK-3β phosphorylation, with subsequent downregulation of β-catenin, suggesting β-catenin signaling modulation as a critical mechanism by which HP exhibits anticancer properties. Our findings suggest HP are of potential therapeutic interest for liver cancer treatment.”