“Beta-Caryophyllene (BCP), a naturally occurring sesquiterpene abundantly found in cloves, hops, and cannabis, is the active candidate of a relatively new group of vascular-inhibiting compounds that aim to block existing tumor blood vessels.
“Beta-Caryophyllene (BCP), a naturally occurring sesquiterpene abundantly found in cloves, hops, and cannabis, is the active candidate of a relatively new group of vascular-inhibiting compounds that aim to block existing tumor blood vessels.
Previously, we have reported the anti-cancer properties of BCP by utilizing a series of in-vitro anti-tumor-related assays using human colorectal carcinoma cells. The present study aimed to investigate the effects of BCP on in-vitro, ex-vivo, and in-vivo models of anti-angiogenic assays and evaluate its anti-cancer activity in xenograft tumor (both ectopic and orthotopic) mice models of human colorectal cancer.
BCP showed a remarkable reduction in tumor size and fluorescence molecular tomography signal intensity in all the mice treated with BCP, in a dose-dependent relationship, in ectopic and orthotopic tumor xenograft models, respectively. The histological analysis of the tumor from BCP-treated mice revealed a clear reduction of the density of vascularization. In addition, BCP induced apoptosis through downregulation of HSP60, HTRA, survivin, and XIAP, along with the upregulation of p21 expressions.
These results suggest that BCP acts at multiple stages of angiogenesis and could be used as a promising therapeutic candidate to halt the growth of colorectal tumor cells.”
https://www.mdpi.com/1422-0067/22/19/10550
“β-caryophyllene (BCP) is a common constitute of the essential oils of numerous spice, food plants and major component in Cannabis.” http://www.ncbi.nlm.nih.gov/pubmed/23138934

 “Cannabidiol (CBD), the major non-psychoactive compound found in cannabis, is frequently used both as a nutraceutical and therapeutic.
“Cannabidiol (CBD), the major non-psychoactive compound found in cannabis, is frequently used both as a nutraceutical and therapeutic. 
 “This study aimed to obtain and characterize extracted hemp oil enriched in cannabidiol (CBD) by decarboxylation of cannabidiolic acid (CBDA) and to give new insights into its antioxidant and anticancer effects.
“This study aimed to obtain and characterize extracted hemp oil enriched in cannabidiol (CBD) by decarboxylation of cannabidiolic acid (CBDA) and to give new insights into its antioxidant and anticancer effects.  “The cannabinoid, cannabidiol (CBD), is part of the plant’s natural defense system that when given to animals has many useful medicinal properties, including activity against cancer cells, modulation of the immune system, and efficacy in epilepsy.
“The cannabinoid, cannabidiol (CBD), is part of the plant’s natural defense system that when given to animals has many useful medicinal properties, including activity against cancer cells, modulation of the immune system, and efficacy in epilepsy.  “Foodborne protein hydrolysates exhibit biological activity that may be therapeutic in a number of human disease settings. Hemp peptides (HP) generated by controlled hydrolysis of hemp proteins have a number of health benefits and are of pharmaceutical value. In the present study, we produce small molecular weight HP from hemp seed and investigate its anticancer properties in Hep3B human liver cancer cells. We demonstrate that HP treatment increased apoptosis, reduced cell viability, and reduced cell migration in Hep3B human liver cancer cells without affecting the normal liver cell line L02. We correlate these phenotypes with increased cellular ROS levels, upregulation of cleaved caspase 3 and Bad, and downregulation of antiapoptotic Bcl-2. HP treatment led to increased Akt and GSK-3β phosphorylation, with subsequent downregulation of β-catenin, suggesting β-catenin signaling modulation as a critical mechanism by which HP exhibits anticancer properties. Our findings suggest HP are of potential therapeutic interest for liver cancer treatment.”
“Foodborne protein hydrolysates exhibit biological activity that may be therapeutic in a number of human disease settings. Hemp peptides (HP) generated by controlled hydrolysis of hemp proteins have a number of health benefits and are of pharmaceutical value. In the present study, we produce small molecular weight HP from hemp seed and investigate its anticancer properties in Hep3B human liver cancer cells. We demonstrate that HP treatment increased apoptosis, reduced cell viability, and reduced cell migration in Hep3B human liver cancer cells without affecting the normal liver cell line L02. We correlate these phenotypes with increased cellular ROS levels, upregulation of cleaved caspase 3 and Bad, and downregulation of antiapoptotic Bcl-2. HP treatment led to increased Akt and GSK-3β phosphorylation, with subsequent downregulation of β-catenin, suggesting β-catenin signaling modulation as a critical mechanism by which HP exhibits anticancer properties. Our findings suggest HP are of potential therapeutic interest for liver cancer treatment.” “Colorectal cancer (CRC) is between the top three occurring cancers worldwide. The anticancer effects of Cannabinoid receptor 2 (CB
“Colorectal cancer (CRC) is between the top three occurring cancers worldwide. The anticancer effects of Cannabinoid receptor 2 (CB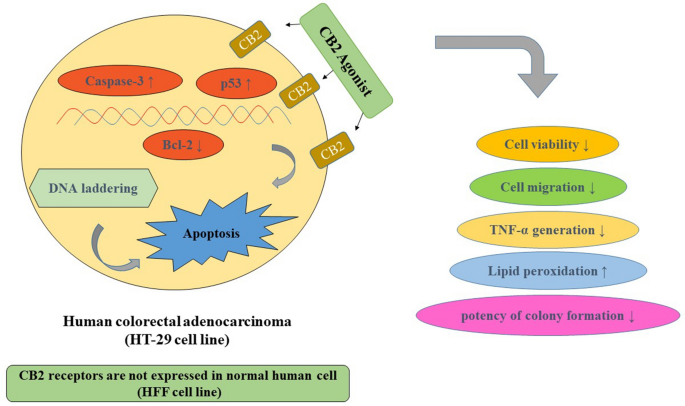
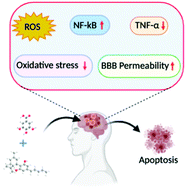
 “Glioblastoma multiforme (GBM) is the most lethal subtype of glioma.
“Glioblastoma multiforme (GBM) is the most lethal subtype of glioma.  “Cannabidiol (CBD) has been shown to slow cancer cell growth and is toxic to human glioblastoma cell lines. Thus, CBD could be an effective therapeutic for glioblastoma.
“Cannabidiol (CBD) has been shown to slow cancer cell growth and is toxic to human glioblastoma cell lines. Thus, CBD could be an effective therapeutic for glioblastoma.