“Introduction: Cannabidiol (CBD) can be isolated from Cannabis sativa L. or synthetically produced. The aim of this study was to compare the in vitro effects of purified natural and synthetic CBD to establish any pharmacological differences or superiority between sources.
Conclusion: Our results suggest that there is no pharmacological difference in vitro in the antiproliferative, anti-inflammatory, or permeability effects of purified natural versus synthetic CBD. The purity and reliability of CBD samples, as well as the ultimate pharmaceutical preparation, should all be considered above the starting source of CBD in the development of new CBD medicines.
This study demonstrates for the first time that the anticancer, neuroprotective, and intestinal barrier protective properties of purified CBD are similar regardless of the source from which CBD is derived. From a pharmacological perspective, where a molecular target is implicated (i.e., 5HT1A in stroke and CB1 in gut permeability), the effects of CBD were similar. This suggests that any beneficial effects that could be achieved in a clinical setting for purified CBD are likely to be similar at a pharmacodynamic level.”
https://www.karger.com/Article/FullText/517120
“Study finds no in-vitro pharmacological difference in the antiproliferative, anti-inflammatory, or permeability effects of purified natural versus synthetic CBD”

 “Glioblastoma multiforme (GBM) is the most lethal subtype of glioma.
“Glioblastoma multiforme (GBM) is the most lethal subtype of glioma. 
 “Melanoma causes the highest number of skin cancer-related deaths worldwide. New treatment methods are essential for the management of this life-threatening disease.
“Melanoma causes the highest number of skin cancer-related deaths worldwide. New treatment methods are essential for the management of this life-threatening disease. “The cannabinoid receptor subtype 2 (CB2R) represents an interesting and new therapeutic target for its involvement in the first steps of neurodegeneration as well as in cancer onset and progression.
“The cannabinoid receptor subtype 2 (CB2R) represents an interesting and new therapeutic target for its involvement in the first steps of neurodegeneration as well as in cancer onset and progression.
 “Objective: Cannabinoids are able to reduce tumor growth in xenograft models, but their therapeutic potential as anti-cancer drugs in humans is unclear yet. In vitro studies of the effect of cannabinoids on cancer cells are often carried out in absence of serum or in low serum concentration (i.e. 0.5% serum), conditions that limit cellular growth and therefore can increase the response of cells to additional challenges such as the presence of cannabinoids. However, the tumor microenvironment can be teaming with growth factors. In this study we assessed the viability and proliferation of cancer cells treated with cannabidiol in presence of a serum concentration that commonly sustains cell growth (10% serum).
“Objective: Cannabinoids are able to reduce tumor growth in xenograft models, but their therapeutic potential as anti-cancer drugs in humans is unclear yet. In vitro studies of the effect of cannabinoids on cancer cells are often carried out in absence of serum or in low serum concentration (i.e. 0.5% serum), conditions that limit cellular growth and therefore can increase the response of cells to additional challenges such as the presence of cannabinoids. However, the tumor microenvironment can be teaming with growth factors. In this study we assessed the viability and proliferation of cancer cells treated with cannabidiol in presence of a serum concentration that commonly sustains cell growth (10% serum).
 “In this study, we investigated the effects of exposition to IC50 dose for 24 h of a new synthetic cannabinoid (CB83) and of phytocannabinoids Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) on HT-29 colorectal carcinoma cells. Cell viability and proliferative activity evaluated using the MTT, lactate dehydrogenase (LDH), and CyQUANT assays showed that cell viability was significantly affected when CB83, THC, and CBD were administered to cells.
“In this study, we investigated the effects of exposition to IC50 dose for 24 h of a new synthetic cannabinoid (CB83) and of phytocannabinoids Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) on HT-29 colorectal carcinoma cells. Cell viability and proliferative activity evaluated using the MTT, lactate dehydrogenase (LDH), and CyQUANT assays showed that cell viability was significantly affected when CB83, THC, and CBD were administered to cells.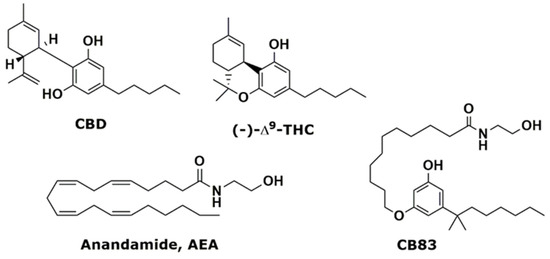
 “The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.
“The intraperitoneal administration of chemotherapeutics has emerged as a potential route in ovarian cancer treatment. Nanoparticles as carriers for these agents could be interesting by increasing the retention of chemotherapeutics within the peritoneal cavity. Moreover, nanoparticles could be internalised by cancer cells and let the drug release near the biological target, which could increase the anticancer efficacy.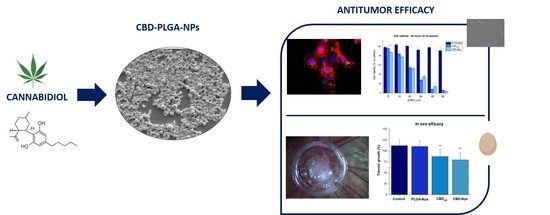
 “Cannabidiol (CBD) has emerged as a potential agent for breast cancer management.
“Cannabidiol (CBD) has emerged as a potential agent for breast cancer management.