 “Coronavirus disease (COVID-19), caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an ongoing pandemic and presents a public health emergency. It has affected millions of people and continues to affect more, despite tremendous social preventive measures. Identifying candidate drugs for the prevention and treatment of COVID-19 is crucial. The pathogenesis and the complications with advanced infection mainly involve an immune-inflammatory cascade. Therefore, therapeutic strategy relies on suppressing infectivity and inflammation, along with immune modulation.
“Coronavirus disease (COVID-19), caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an ongoing pandemic and presents a public health emergency. It has affected millions of people and continues to affect more, despite tremendous social preventive measures. Identifying candidate drugs for the prevention and treatment of COVID-19 is crucial. The pathogenesis and the complications with advanced infection mainly involve an immune-inflammatory cascade. Therefore, therapeutic strategy relies on suppressing infectivity and inflammation, along with immune modulation.
One of the most promising therapeutic targets for the modulation of immune-inflammatory responses is the endocannabinoid system, particularly the activation of cannabinoid type 2 receptors (CB2R), a G-protein coupled receptor which mediates the anti-inflammatory properties by modulating numerous signaling pathways. To pharmacologically activate the CB2 receptors, a naturally occurring cannabinoid ligand, beta-caryophyllene (BCP), received attention due to its potent anti-inflammatory, antiviral, and immunomodulatory properties. BCP is recognized as a full selective functional agonist on CB2 receptors and produces therapeutic effects by activating CB2 and the nuclear receptors, peroxisome proliferator-activated receptors (PPARs).
BCP is regarded as the first dietary cannabinoid with abundant presence across cannabis and non-cannabis plants, including spices and other edible plants. BCP showed tissue protective properties and favorably modulates numerous signaling pathways and inhibits inflammatory mediators, including cytokines, chemokines, adhesion molecules, prostanoids, and eicosanoids. Based on its pharmacological properties, molecular mechanisms, and the therapeutic potential of BCP as an immunomodulator, anti-inflammatory, organ-protective, and antiviral, we hypothesize that BCP could be a promising therapeutic and/or preventive candidate to target the triad of infection, immunity, and inflammation in COVID-19. In line with numerous studies that proposed the potential of cannabinoids in COVID-19,
BCP may be a novel candidate compound for pharmaceutical and nutraceutical development due to its unique functional receptor selectivity, wide availability and accessibility, dietary bioavailability, nonpsychoactivity, and negligible toxicity along with druggable properties, including favorable pharmacokinetic and physicochemical properties. Based on reasonable pharmacological mechanisms and therapeutic properties, we speculate that BCP has potential to be investigated against COVID-19 and will inspire further preclinical and clinical studies.”
https://pubmed.ncbi.nlm.nih.gov/34054510/
“Over the past few months, it has been suggested that modulation of the endocannabinoid system by cannabinoids, including cannabidiol, could be useful in prophylaxis and treatment of COVID-19 and may improve prognosis. Recently, extract of Cannabis sativa containing phytocannabinoids and terpenes were shown to modulate the inflammatory mediators in alveolar epithelial cells (A549) in COVID-19-associated inflammation and suggested that the phytocannabinoid mix formulation exerted better activity in comparison with individual fractions from cannabis. Many cannabinoids, including cannabidiol, have been suggested for their possible potential as preventive agents or therapeutic adjuvants with other agents in targeting the trinity of infection, inflammation, and immunity in COVID-19.”
“β-caryophyllene (BCP) is a common constitute of the essential oils of numerous spice, food plants and major component in Cannabis.” http://www.ncbi.nlm.nih.gov/pubmed/23138934
 “Objective: Joint injury-induced perturbations to the endocannabinoid system (ECS), a regulator of both inflammation and nociception, remain largely uncharacterized. We employed a mouse model of ACL rupture to assess alterations to nociception, inflammation, and the ECS while using in vitro models to determine whether CB2 agonism can mitigate inflammatory signaling in macrophages and fibroblast-like synoviocytes (FLS).
“Objective: Joint injury-induced perturbations to the endocannabinoid system (ECS), a regulator of both inflammation and nociception, remain largely uncharacterized. We employed a mouse model of ACL rupture to assess alterations to nociception, inflammation, and the ECS while using in vitro models to determine whether CB2 agonism can mitigate inflammatory signaling in macrophages and fibroblast-like synoviocytes (FLS).
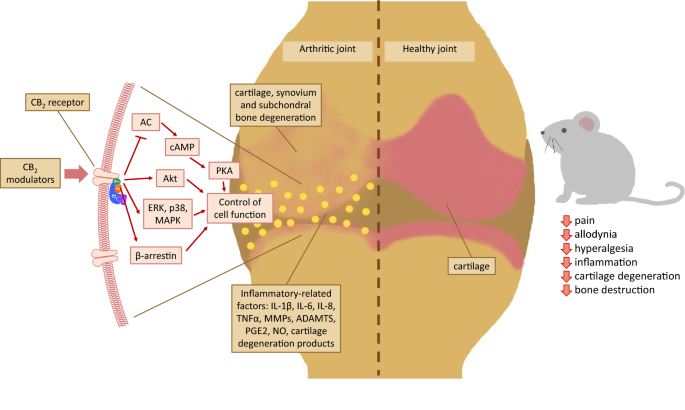 “Over the last several decades, the percentage of patients suffering from different forms of arthritis has increased due to the ageing population and the increasing risk of civilization diseases, e.g. obesity, which contributes to arthritis development. Osteoarthritis and rheumatoid arthritis are estimated to affect 50-60% of people over 65 years old and cause serious health and economic problems. Currently, therapeutic strategies are limited and focus mainly on pain attenuation and maintaining joint functionality. First-line therapies are nonsteroidal anti-inflammatory drugs; in more advanced stages, stronger analgesics, such as opioids, are required, and in the most severe cases, joint arthroplasty is the only option to ensure joint mobility.
“Over the last several decades, the percentage of patients suffering from different forms of arthritis has increased due to the ageing population and the increasing risk of civilization diseases, e.g. obesity, which contributes to arthritis development. Osteoarthritis and rheumatoid arthritis are estimated to affect 50-60% of people over 65 years old and cause serious health and economic problems. Currently, therapeutic strategies are limited and focus mainly on pain attenuation and maintaining joint functionality. First-line therapies are nonsteroidal anti-inflammatory drugs; in more advanced stages, stronger analgesics, such as opioids, are required, and in the most severe cases, joint arthroplasty is the only option to ensure joint mobility.  “Coronavirus disease (COVID-19), caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an ongoing pandemic and presents a public health emergency. It has affected millions of people and continues to affect more, despite tremendous social preventive measures. Identifying candidate drugs for the prevention and treatment of COVID-19 is crucial. The pathogenesis and the complications with advanced infection mainly involve an immune-inflammatory cascade. Therefore, therapeutic strategy relies on suppressing infectivity and inflammation, along with immune modulation.
“Coronavirus disease (COVID-19), caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an ongoing pandemic and presents a public health emergency. It has affected millions of people and continues to affect more, despite tremendous social preventive measures. Identifying candidate drugs for the prevention and treatment of COVID-19 is crucial. The pathogenesis and the complications with advanced infection mainly involve an immune-inflammatory cascade. Therefore, therapeutic strategy relies on suppressing infectivity and inflammation, along with immune modulation.  “Agents targeting the endocannabinoid system (ECS) have gained attention as potential cancer treatments. Given recent evidence that cannabinoid receptor 2 (CB2R) regulates lymphocyte development and inflammation, we performed studies on CB2R in the immune response against melanoma. Analysis of The Cancer Genome Atlas (TCGA) data revealed a strong positive correlation between CB2R expression and survival, as well as B cell infiltration in human melanoma. In a murine melanoma model, CB2R expression reduced the growth of melanoma as well as the B cell frequencies in the tumor microenvironment (TME), compared to CB2R-deficient mice. In depth analysis of tumor-infiltrating B cells using single-cell RNA sequencing suggested a less differentiated phenotype in tumors from
“Agents targeting the endocannabinoid system (ECS) have gained attention as potential cancer treatments. Given recent evidence that cannabinoid receptor 2 (CB2R) regulates lymphocyte development and inflammation, we performed studies on CB2R in the immune response against melanoma. Analysis of The Cancer Genome Atlas (TCGA) data revealed a strong positive correlation between CB2R expression and survival, as well as B cell infiltration in human melanoma. In a murine melanoma model, CB2R expression reduced the growth of melanoma as well as the B cell frequencies in the tumor microenvironment (TME), compared to CB2R-deficient mice. In depth analysis of tumor-infiltrating B cells using single-cell RNA sequencing suggested a less differentiated phenotype in tumors from 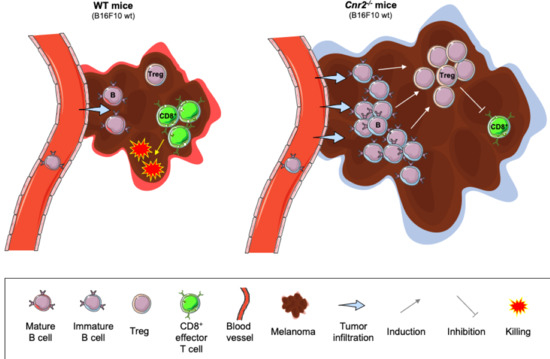
 “Colorectal cancer (CRC) is between the top three occurring cancers worldwide. The anticancer effects of Cannabinoid receptor 2 (CB
“Colorectal cancer (CRC) is between the top three occurring cancers worldwide. The anticancer effects of Cannabinoid receptor 2 (CB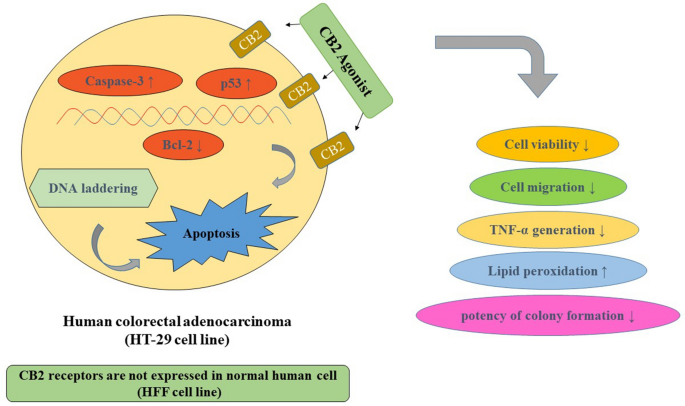
 “The COVID-19 pandemic caused by SARS-CoV-2 is a deadly disease afflicting millions. The pandemic continues affecting population due to nonavailability of drugs and vaccines. The pathogenesis and complications of infection mainly involve hyperimmune-inflammatory responses. Thus, therapeutic strategies rely on repurposing of drugs aimed at reducing infectivity and inflammation and modulate immunity favourably.
“The COVID-19 pandemic caused by SARS-CoV-2 is a deadly disease afflicting millions. The pandemic continues affecting population due to nonavailability of drugs and vaccines. The pathogenesis and complications of infection mainly involve hyperimmune-inflammatory responses. Thus, therapeutic strategies rely on repurposing of drugs aimed at reducing infectivity and inflammation and modulate immunity favourably.
 “(E)-β-caryophyllene (BCP) is a bicyclic sesquiterpene widely distributed in the plant kingdom, where it contributes a unique aroma to essential oils and has a pivotal role in the survival and evolution of higher plants.
“(E)-β-caryophyllene (BCP) is a bicyclic sesquiterpene widely distributed in the plant kingdom, where it contributes a unique aroma to essential oils and has a pivotal role in the survival and evolution of higher plants. “The cannabinoid receptor subtype 2 (CB2R) represents an interesting and new therapeutic target for its involvement in the first steps of neurodegeneration as well as in cancer onset and progression.
“The cannabinoid receptor subtype 2 (CB2R) represents an interesting and new therapeutic target for its involvement in the first steps of neurodegeneration as well as in cancer onset and progression.
 “Uterus transplantation is a complex surgical procedure. Uterine ischemia/reperfusion (IR) damage occurring in this process may cause loss of function in the uterus. Cell damage must be prevented for a healthy uterine function and successful transplantation.
“Uterus transplantation is a complex surgical procedure. Uterine ischemia/reperfusion (IR) damage occurring in this process may cause loss of function in the uterus. Cell damage must be prevented for a healthy uterine function and successful transplantation. “Graft versus host disease (GVHD) pathophysiology is a complex interplay between cells that comprise the adaptive and innate arms of the immune system. Effective prophylactic strategies are therefore contingent upon approaches that address contributions from both immune cell compartments.
“Graft versus host disease (GVHD) pathophysiology is a complex interplay between cells that comprise the adaptive and innate arms of the immune system. Effective prophylactic strategies are therefore contingent upon approaches that address contributions from both immune cell compartments.