“Rheumatoid arthritis (RA) is a painful chronic autoimmune disease affecting the joints. Its first-line therapy, Methotrexate (MTX), although effective in ameliorating the progress of the disease, induces hepatotoxicity over long-term usage. Thus, seeking natural compounds with fewer side effects could be an alternative therapeutic approach. This study aimed to investigate the anti-inflammatory, antiarthritic, and antioxidative effects of synthetic trans-Δ9-tetrahydrocannabinol (Δ9-THC) dissolved in sesame oil (Dronabinol) against MTX in adjuvant-induced arthritis (AIA) rat model. Daily oral administration of Δ9-THC/sesame oil, over a period of 21 days, was well tolerated in arthritic rats with no particular psychoactive side effects. It markedly attenuated the severity of clinical manifestations, recovered the histopathological changes in tibiotarsal joints, and repressed the splenomegaly in arthritic rats. Δ9-THC/sesame oil therapy showed similar effects to MTX in neutralizing the inflammatory process of AIA, through attenuating erythrocyte sedimentation rate (ESR) scores and proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin 1-beta (IL-1β), and interleukin-6 (IL-6) levels, to normal values. As opposed to MTX, this natural combination markedly protected the liver of arthritic rats and downregulated the induced oxidative stress by increasing the antioxidant defense system such as activities of catalase and superoxide dismutase (SOD) and levels of glutathione (GSH). These results suggest promising effects for the clinical use of Δ9-THC/sesame oil therapy in alleviating arthritic clinical signs as well as arthritis-induced liver injury.”
https://pubmed.ncbi.nlm.nih.gov/30046349/
“Dronabinol (Δ9-THC in sesame oil) is usually used to treat nausea and vomiting caused by chemotherapy or weight loss and loss of appetite in AIDS patients, yet, to the best of our knowledge, this is the first study that proves the antiarthritic and antioxidative effects of this combination in an experimental model of RA with a hepatoprotective effect against arthritis-induced liver injury compared to commonly used antirheumatic drug (MTX).”
https://www.hindawi.com/journals/ecam/2018/9365464/

 “Uterus transplantation is a complex surgical procedure. Uterine ischemia/reperfusion (IR) damage occurring in this process may cause loss of function in the uterus. Cell damage must be prevented for a healthy uterine function and successful transplantation.
“Uterus transplantation is a complex surgical procedure. Uterine ischemia/reperfusion (IR) damage occurring in this process may cause loss of function in the uterus. Cell damage must be prevented for a healthy uterine function and successful transplantation. “In this proof-of-concept study, the antioxidant activity of phytocannabinoids, namely cannabidiol (CBD) and Δ9- tetrahydrocannabinol (THC), were investigated using an in vitro system of differentiated human neuronal SY-SH5Y cells.
“In this proof-of-concept study, the antioxidant activity of phytocannabinoids, namely cannabidiol (CBD) and Δ9- tetrahydrocannabinol (THC), were investigated using an in vitro system of differentiated human neuronal SY-SH5Y cells.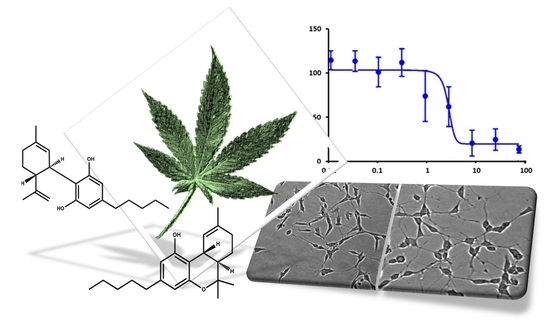
 “Cannabinoids are the chemical compounds with a high affinity for cannabinoid receptors affecting the central nervous system through the release of neurotransmitters. However, the current knowledge related to the role of such compounds in the regulation of cellular aging is limited. This study aimed to investigate the effect of cannabidiol and tetrahydrocannabinol on the function of aged pancreatic islets.
“Cannabinoids are the chemical compounds with a high affinity for cannabinoid receptors affecting the central nervous system through the release of neurotransmitters. However, the current knowledge related to the role of such compounds in the regulation of cellular aging is limited. This study aimed to investigate the effect of cannabidiol and tetrahydrocannabinol on the function of aged pancreatic islets. “Renal ischemia-reperfusion injury (IRI) is a major cause of acute kidney injury (AKI) and even induces remote organ damage.
“Renal ischemia-reperfusion injury (IRI) is a major cause of acute kidney injury (AKI) and even induces remote organ damage.

