 “The concept of neurons as irreplaceable cells does not hold true today. Experiments and evidence of neurogenesis, also, in the adult brain give hope that some compounds or drugs can enhance this process, helping to reverse the outcomes of diseases or traumas that once were thought to be everlasting.
“The concept of neurons as irreplaceable cells does not hold true today. Experiments and evidence of neurogenesis, also, in the adult brain give hope that some compounds or drugs can enhance this process, helping to reverse the outcomes of diseases or traumas that once were thought to be everlasting.
Cannabinoids, both from natural and artificial origins, already proved to have several beneficial effects (e.g., anti-inflammatory, anti-oxidants and analgesic action), but also capacity to increase neuronal population, by replacing the cells that were lost and/or regenerate a damaged nerve cell.
Neurogenesis is a process which is not highly represented in literature as neuroprotection, though it is as important as prevention of nervous system damage, because it can represent a possible solution when neuronal death is already present, such as in neurodegenerative diseases.
The aim of this review is to resume the experimental evidence of phyto- and synthetic cannabinoids effects on neurogenesis, both in vitro and in vivo, in order to elucidate if they possess also neurogenetic and neurorepairing properties.”
https://pubmed.ncbi.nlm.nih.gov/34684894/
“The current results of cannabinoids effects on neurogenesis are encouraging, and it is expectable that the amount of evidence continues to increase in the future with other experiments.”

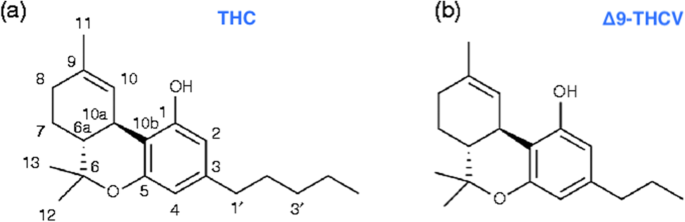 “Δ9-Tetrahydrocannabivarin (THCV) is a cannabis-derived compound with unique properties that set it apart from the more common cannabinoids, such as Δ9-tetrahydrocannabinol (THC). The main advantage of THCV over THC is the lack of psychoactive effects.
“Δ9-Tetrahydrocannabivarin (THCV) is a cannabis-derived compound with unique properties that set it apart from the more common cannabinoids, such as Δ9-tetrahydrocannabinol (THC). The main advantage of THCV over THC is the lack of psychoactive effects.  “Background and purpose:
“Background and purpose:  “Cannabinoids have long been used for their psychotropic and possible medical properties of symptom relief. In the past few years, a vast literature shows that cannabinoids are neuroprotective under different pathological situations.
“Cannabinoids have long been used for their psychotropic and possible medical properties of symptom relief. In the past few years, a vast literature shows that cannabinoids are neuroprotective under different pathological situations. “While activation of cannabinoid (CB2) receptors has been shown to be neuroprotective, no studies have examined whether this neuroprotection is directed at cerebral arterioles and no studies have examined whether activation of CB2 receptors can rescue cerebrovascular dysfunction during a chronic disease state such as type 1 diabetes (T1D).
“While activation of cannabinoid (CB2) receptors has been shown to be neuroprotective, no studies have examined whether this neuroprotection is directed at cerebral arterioles and no studies have examined whether activation of CB2 receptors can rescue cerebrovascular dysfunction during a chronic disease state such as type 1 diabetes (T1D). “The prevalence of mild traumatic brain injury is highest amongst the adolescent population and can lead to complications including neuroinflammation and excitotoxicity.
“The prevalence of mild traumatic brain injury is highest amongst the adolescent population and can lead to complications including neuroinflammation and excitotoxicity. “Alzheimer’s disease (AD) is a multifactorial neurodegenerative disorder linked to various converging toxic mechanisms. Evidence suggests that hyperglycemia induces oxidative stress, mitochondrial dysfunction, inflammation and excitotoxicity, all of which play important roles in the onset and progression of AD pathogenesis.
“Alzheimer’s disease (AD) is a multifactorial neurodegenerative disorder linked to various converging toxic mechanisms. Evidence suggests that hyperglycemia induces oxidative stress, mitochondrial dysfunction, inflammation and excitotoxicity, all of which play important roles in the onset and progression of AD pathogenesis. “Neurological disorders such as neurodegenerative diseases or traumatic brain injury are associated with cognitive, motor and behavioural changes that influence the quality of life of the patients. Although different therapeutic strategies have been developed and tried until now to decrease the neurological decline, no treatment has been found to cure these pathologies.
“Neurological disorders such as neurodegenerative diseases or traumatic brain injury are associated with cognitive, motor and behavioural changes that influence the quality of life of the patients. Although different therapeutic strategies have been developed and tried until now to decrease the neurological decline, no treatment has been found to cure these pathologies.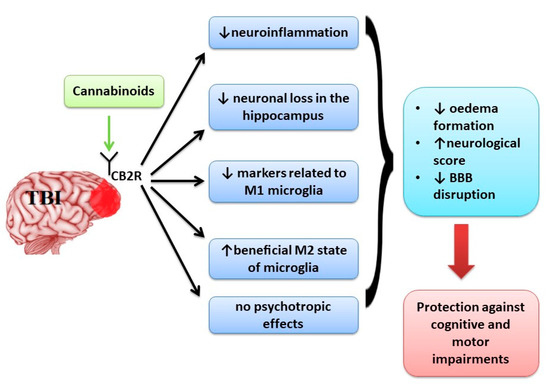
 “New neuroprotective treatments of natural origin are being investigated. Both, plant extracts and isolated compounds have shown bioactive effects.
“New neuroprotective treatments of natural origin are being investigated. Both, plant extracts and isolated compounds have shown bioactive effects.
 “Parkinson’s Disease (PD) is currently the most rapid growing neurodegenerative disease and over the past generation, its global burden has more than doubled. The onset of PD can arise due to environmental, sporadic or genetic factors. Nevertheless, most PD cases have an unknown etiology.
“Parkinson’s Disease (PD) is currently the most rapid growing neurodegenerative disease and over the past generation, its global burden has more than doubled. The onset of PD can arise due to environmental, sporadic or genetic factors. Nevertheless, most PD cases have an unknown etiology.