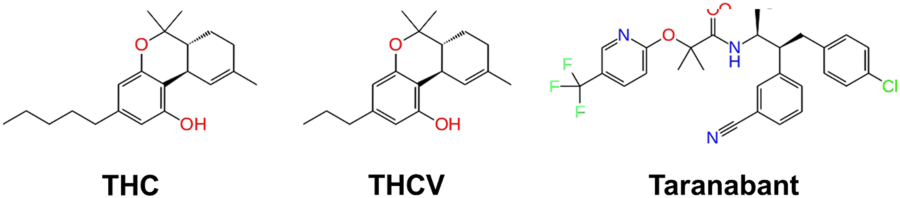
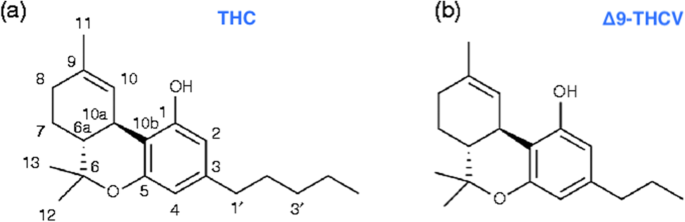
 “Δ9-Tetrahydrocannabivarin (THCV) is a cannabis-derived compound with unique properties that set it apart from the more common cannabinoids, such as Δ9-tetrahydrocannabinol (THC). The main advantage of THCV over THC is the lack of psychoactive effects. In rodent studies, THCV decreases appetite, increases satiety, and up-regulates energy metabolism, making it a clinically useful remedy for weight loss and management of obesity and type 2 diabetic patients. The distinctions between THCV and THC in terms of glycemic control, glucose metabolism, and energy regulation have been demonstrated in previous studies. Also, the effect of THCV on dyslipidemia and glycemic control in type 2 diabetics showed reduced fasting plasma glucose concentration when compared to a placebo group. In contrast, THC is indicated in individuals with cachexia. However, the uniquely diverse properties of THCV provide neuroprotection, appetite suppression, glycemic control, and reduced side effects, etc.; therefore, making it a potential priority candidate for the development of clinically useful therapies in the future. Hopefully, THCV could provide an optional platform for the treatment of life-threatening diseases.”
“Δ9-Tetrahydrocannabivarin (THCV) is a cannabis-derived compound with unique properties that set it apart from the more common cannabinoids, such as Δ9-tetrahydrocannabinol (THC). The main advantage of THCV over THC is the lack of psychoactive effects. In rodent studies, THCV decreases appetite, increases satiety, and up-regulates energy metabolism, making it a clinically useful remedy for weight loss and management of obesity and type 2 diabetic patients. The distinctions between THCV and THC in terms of glycemic control, glucose metabolism, and energy regulation have been demonstrated in previous studies. Also, the effect of THCV on dyslipidemia and glycemic control in type 2 diabetics showed reduced fasting plasma glucose concentration when compared to a placebo group. In contrast, THC is indicated in individuals with cachexia. However, the uniquely diverse properties of THCV provide neuroprotection, appetite suppression, glycemic control, and reduced side effects, etc.; therefore, making it a potential priority candidate for the development of clinically useful therapies in the future. Hopefully, THCV could provide an optional platform for the treatment of life-threatening diseases.”
“The psychoactive effects of THC in marijuana are the main reasons for its classification as a Schedule I substance, even though it is the THC that the U.S. Food and Drug Administration (FDA) approved for appetite stimulation and weight gain. In contrast to THC, clinical and therapeutic advantages of THCV regarding its lack of psychoactive effects in human studies are of great value in pharmacotherapy. It is envisioned that the unique and diverse characteristics of THCV could be explored for further development into clinically useful medicines for the treatment of life-threatening diseases.”
https://jcannabisresearch.biomedcentral.com/articles/10.1186/s42238-020-0016-7

 “Cannabis is an annual plant with a long history of use as food, feed, fiber, oil, medicine, and narcotics. Despite realizing its true value, it has not yet found its true place. Cannabis has had a long history with many ups and downs, and now it is our turn to promote it.
“Cannabis is an annual plant with a long history of use as food, feed, fiber, oil, medicine, and narcotics. Despite realizing its true value, it has not yet found its true place. Cannabis has had a long history with many ups and downs, and now it is our turn to promote it.
 “The antioxidant and CB2 receptor agonist properties of Δ9-tetrahydrocannabivarin (Δ9-THCV) afforded neuroprotection in experimental Parkinson’s disease (PD), whereas its CB1 receptor antagonist profile at doses lower than 5 mg/kg caused anti-hypokinetic effects.
“The antioxidant and CB2 receptor agonist properties of Δ9-tetrahydrocannabivarin (Δ9-THCV) afforded neuroprotection in experimental Parkinson’s disease (PD), whereas its CB1 receptor antagonist profile at doses lower than 5 mg/kg caused anti-hypokinetic effects. “The cellular microenvironment plays a critical role in the maintenance of bone marrow-derived mesenchymal stem cells (BM-MSCs) and their subsequent cell lineage differentiation. Recent studies suggested that individuals with adipocyte-related metabolic disorders have altered function and adipogenic potential of adipose stem cell subpopulations, primarily BM-MSCs, increasing the risk of heart attack, stroke or diabetes.
“The cellular microenvironment plays a critical role in the maintenance of bone marrow-derived mesenchymal stem cells (BM-MSCs) and their subsequent cell lineage differentiation. Recent studies suggested that individuals with adipocyte-related metabolic disorders have altered function and adipogenic potential of adipose stem cell subpopulations, primarily BM-MSCs, increasing the risk of heart attack, stroke or diabetes.
 “Substance use disorder (SUD) is a major public health crisis worldwide, and effective treatment options are limited.
“Substance use disorder (SUD) is a major public health crisis worldwide, and effective treatment options are limited. “Both types of
“Both types of