 “Cannabis is an annual plant with a long history of use as food, feed, fiber, oil, medicine, and narcotics. Despite realizing its true value, it has not yet found its true place. Cannabis has had a long history with many ups and downs, and now it is our turn to promote it.
“Cannabis is an annual plant with a long history of use as food, feed, fiber, oil, medicine, and narcotics. Despite realizing its true value, it has not yet found its true place. Cannabis has had a long history with many ups and downs, and now it is our turn to promote it.
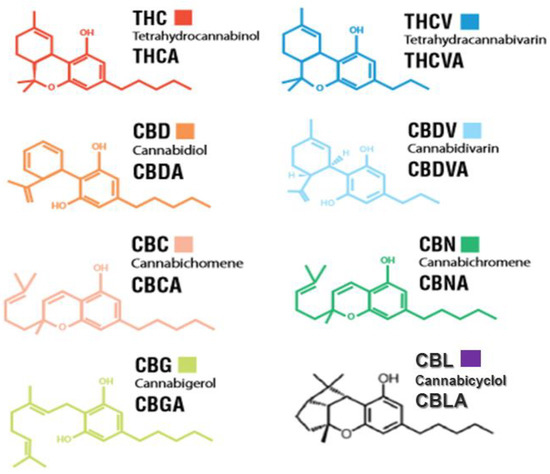
Cannabis contains approximately 600 identified and many yet unidentified potentially useful compounds. Cannabinoids, phenolic compounds, terpenoids, and alkaloids are some of the secondary metabolites present in cannabis. However, among a plethora of unique chemical compounds found in this plant, the most important ones are phytocannabinoids (PCs).
Over hundreds of 21-22-carbon compounds exclusively produce in cannabis glandular hairs through either polyketide and or deoxyxylulose phosphate/methylerythritol phosphate (DOXP/MEP) pathways. Trans-Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) are those that first come to mind while talking about cannabis. Nevertheless, despite the low concentration, cannabinol (CBN), cannabigerol (CBG), cannabichromene (CBC), tetrahydrocannabivarin (THCV), cannabidivarin (CBDV), cannabinodiol (CBND), and cannabinidiol (CBDL) may have potentially some medical effects.
PCs and endocannabinoids (ECs) mediate their effects mainly through CB1 and CB2 receptors. Despite all concerns regarding cannabis, nobody can ignore the use of cannabinoids as promising tonic, analgesic, antipyretic, antiemetic, anti-inflammatory, anti-epileptic, anticancer agents, which are effective for pain relief, depression, anxiety, sleep disorders, nausea and vomiting, multiple sclerosis, cardiovascular disorders, and appetite stimulation.
The scientific community and public society have now increasingly accepted cannabis specifically hemp as much more than a recreational drug. There are growing demands for cannabinoids, mainly CBD, with many diverse therapeutic and nutritional properties in veterinary or human medicine. The main objective of this review article is to historically summarize findings concerning cannabinoids, mainly THC and CBD, towards putting these valuable compounds into food, feed and health baskets and current and future trends in the consumption of products derived from cannabis.”
https://pubmed.ncbi.nlm.nih.gov/32899626/
https://www.mdpi.com/1420-3049/25/18/4036

 “Glioma-related epilepsy significantly impact on patients’ quality of life, and can often be difficult to treat. Seizures cause significant morbidity for example neurocognitive deterioration, which may result from seizures themselves or due to adverse effects from antiepileptic drugs. Management of tumour with surgery, radiotherapy and chemotherapy may contribute to seizure control, but tumour related epilepsy is often refractory despite adequate treatment with standard anti-epileptic medications. Given the increasing interest in medicinal cannabis (or
“Glioma-related epilepsy significantly impact on patients’ quality of life, and can often be difficult to treat. Seizures cause significant morbidity for example neurocognitive deterioration, which may result from seizures themselves or due to adverse effects from antiepileptic drugs. Management of tumour with surgery, radiotherapy and chemotherapy may contribute to seizure control, but tumour related epilepsy is often refractory despite adequate treatment with standard anti-epileptic medications. Given the increasing interest in medicinal cannabis (or  “Drug-resistant seizures are life-threatening and contribute to sustained hospitalization.
“Drug-resistant seizures are life-threatening and contribute to sustained hospitalization. “Cannabis sativa (cannabis) is one of the oldest plants cultivated by men. Cannabidiol (CBD) is the major non-psychomimetic compound derived from cannabis. It has been proposed to have a therapeutic potential over a wide range of neuropsychiatric disorders.
“Cannabis sativa (cannabis) is one of the oldest plants cultivated by men. Cannabidiol (CBD) is the major non-psychomimetic compound derived from cannabis. It has been proposed to have a therapeutic potential over a wide range of neuropsychiatric disorders.





/cdn.vox-cdn.com/uploads/chorus_image/image/54932609/2928600995_24caa84411_o.0.0.jpg)