 “Anxiety and depressive disorders are highly prevalent. Patients are increasingly using medicinal cannabis products to treat these disorders, but little is known about the effects of medicinal cannabis use on symptoms of anxiety and depression.
“Anxiety and depressive disorders are highly prevalent. Patients are increasingly using medicinal cannabis products to treat these disorders, but little is known about the effects of medicinal cannabis use on symptoms of anxiety and depression.
The aim of the present observational study was to assess general health in medicinal cannabis users and non-using controls with anxiety and/or depression.
Results: Medicinal cannabis use was associated with lower self-reported depression, but not anxiety, at baseline. Medicinal cannabis users also reported superior sleep, quality of life, and less pain on average. Initiation of medicinal cannabis during the follow-up period was associated with significantly decreased anxiety and depressive symptoms, an effect that was not observed in Controls that never initiated cannabis use.
Conclusions: Medicinal cannabis use may reduce anxiety and depressive symptoms in clinically anxious and depressed populations. Future placebo-controlled studies are necessary to replicate these findings and to determine the route of administration, dose, and product formulation characteristics to optimize clinical outcomes.”
https://www.frontiersin.org/articles/10.3389/fpsyt.2021.729800/full
“Johns Hopkins: New Study Backs Claims That Cannabis Can Reduce Anxiety And Depression” https://finance.yahoo.com/news/johns-hopkins-study-backs-claims-145005658.html
“Report Shows Cannabis is Effective in Treating Anxiety, Depression” https://www.legalreader.com/report-shows-cannabis-is-effective-in-treating-anxiety-depression/

 “Importance:
“Importance:  “The potential therapeutic use of some Cannabis sativa plant compounds has been attracting great interest, especially for managing neuropsychiatric disorders due to the relative lack of efficacy of the current treatments.
“The potential therapeutic use of some Cannabis sativa plant compounds has been attracting great interest, especially for managing neuropsychiatric disorders due to the relative lack of efficacy of the current treatments.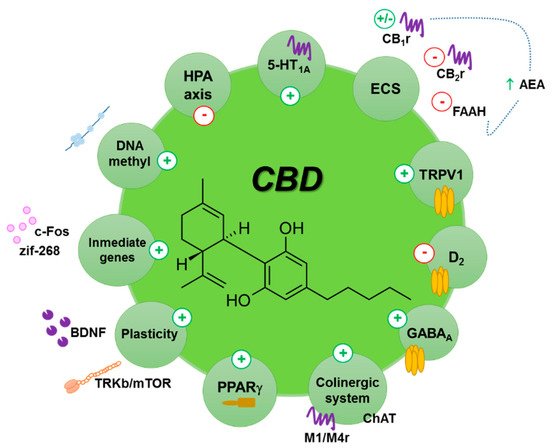
 “Cannabidiol (CBD) is a non-psychoactive phytocannabinoid known for its beneficial effects including antioxidant and anti-inflammatory properties. Moreover, CBD is a compound with antidepressant, anxiolytic, anticonvulsant and antipsychotic effects. Thanks to all these properties, the interest of the scientific community for it has grown.
“Cannabidiol (CBD) is a non-psychoactive phytocannabinoid known for its beneficial effects including antioxidant and anti-inflammatory properties. Moreover, CBD is a compound with antidepressant, anxiolytic, anticonvulsant and antipsychotic effects. Thanks to all these properties, the interest of the scientific community for it has grown. “Scientific research on how consumption of whole, natural Cannabis flower affects low mood and behavioral motivations more generally is largely nonexistent, and few studies to date have measured how common and commercially available Cannabis flower used in vivo may affect the experience of “depression” in real-time.
“Scientific research on how consumption of whole, natural Cannabis flower affects low mood and behavioral motivations more generally is largely nonexistent, and few studies to date have measured how common and commercially available Cannabis flower used in vivo may affect the experience of “depression” in real-time. “The worldwide prevalence of neurological and neurodegenerative disorders, such as depression or Alzheimer’s disease, has spread extensively throughout the last decades, becoming an enormous health issue.
“The worldwide prevalence of neurological and neurodegenerative disorders, such as depression or Alzheimer’s disease, has spread extensively throughout the last decades, becoming an enormous health issue.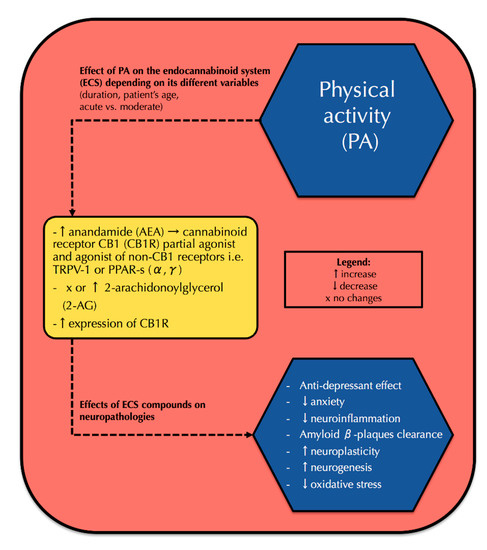
 “The prevalence rates of depression and anxiety are at least two times higher in diabetic patients, increasing morbidity and mortality.
“The prevalence rates of depression and anxiety are at least two times higher in diabetic patients, increasing morbidity and mortality.
