
“Glioblastoma (GBM) is the most frequent malignant tumor of the central nervous system in humans with a median survival time of less than 15 months.
∆9-Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best-characterized components of Cannabis sativa plants with modulating effects on cannabinoid receptors 1 and 2 (CB1 and CB2) and on orphan receptors such as GPR18 or GPR55. Previous studies have demonstrated anti-tumorigenic effects of THC and CBD in several tumor entities including GBM, mostly mediated via CB1 or CB2.
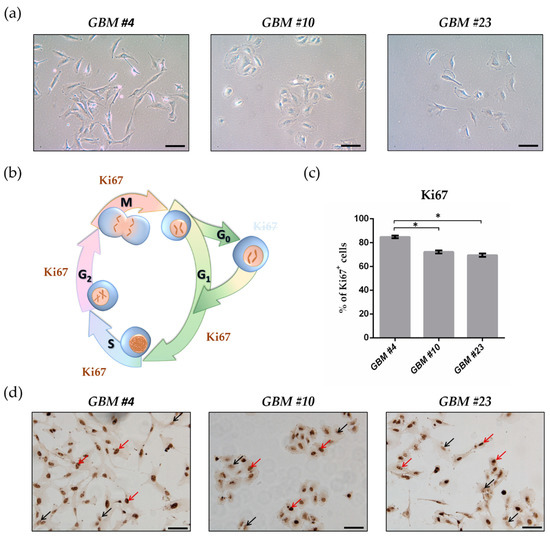
In this study, we investigated the non-CB1/CB2 effects of THC on the cell cycle of GBM cells isolated from human tumor samples.
Cell cycle entry was measured after 24 h upon exposure by immunocytochemical analysis of Ki67 as proliferation marker. The Ki67-reducing effect of THC was abolished in the presence of CBD, whereas CBD alone did not cause any changes. To identify the responsible receptor for THC effects, we first characterized the cells regarding their expression of different cannabinoid receptors: CB1, CB2, GPR18, and GPR55. Secondly, the receptors were pharmacologically blocked by application of their selective antagonists AM281, AM630, O-1918, and CID16020046 (CID), respectively. All examined cells expressed the receptors, but only in presence of the GPR55 antagonist CID was the THC effect diminished. Stimulation with the GPR55 agonist lysophosphatidylinositol (LPI) revealed similar effects as obtained for THC. The LPI effects were also inhibited by CBD and CID, confirming a participation of GPR55 and suggesting its involvement in modifying the cell cycle of patient-derived GBM cells.”
https://pubmed.ncbi.nlm.nih.gov/33802282/
https://www.mdpi.com/2072-6694/13/5/1064

 “Cannabidiol (CBD) is a non-psychoactive phytocannabinoid known for its beneficial effects including antioxidant and anti-inflammatory properties. Moreover, CBD is a compound with antidepressant, anxiolytic, anticonvulsant and antipsychotic effects. Thanks to all these properties, the interest of the scientific community for it has grown.
“Cannabidiol (CBD) is a non-psychoactive phytocannabinoid known for its beneficial effects including antioxidant and anti-inflammatory properties. Moreover, CBD is a compound with antidepressant, anxiolytic, anticonvulsant and antipsychotic effects. Thanks to all these properties, the interest of the scientific community for it has grown. “∆ 9 -Tetrahydrocannabinol (∆9 -THC), the active phytocannabinoid in cannabis, is virtually an adjunct to the endogenous endocannabinoid signaling system.
“∆ 9 -Tetrahydrocannabinol (∆9 -THC), the active phytocannabinoid in cannabis, is virtually an adjunct to the endogenous endocannabinoid signaling system. “Epilepsy contributes to approximately 1% of the global disease burden. By affecting especially young children as well as older persons of all social and racial variety, epilepsy is a present disorder worldwide. Currently, only 65% of epileptic patients can be successfully treated with antiepileptic drugs. For this reason, alternative medicine receives more attention.
“Epilepsy contributes to approximately 1% of the global disease burden. By affecting especially young children as well as older persons of all social and racial variety, epilepsy is a present disorder worldwide. Currently, only 65% of epileptic patients can be successfully treated with antiepileptic drugs. For this reason, alternative medicine receives more attention. “Cannabinoid receptors type 1 (CB1) and 2 (CB2) are widely expressed in the human body, and are attractive drug targets in the prevention and management of central nervous system (CNS) and immune system dysfunction, respectively. Recent breakthroughs in the structural elucidation of cannabinoid receptors and their signaling complexes with G proteins, provide the important molecular basis of ligand-receptor interactions, activation and signaling mechanism, which will facilitate the next-generation drug design and the precise modulation of the endocannabinoid system. Here, we provide an overview on the structural features of cannabinoid receptors in different functional states and the diverse ligand binding modes. The major challenges and new strategies for future therapeutic applications targeting the endocannabinoid system (ECS) are also discussed.”
“Cannabinoid receptors type 1 (CB1) and 2 (CB2) are widely expressed in the human body, and are attractive drug targets in the prevention and management of central nervous system (CNS) and immune system dysfunction, respectively. Recent breakthroughs in the structural elucidation of cannabinoid receptors and their signaling complexes with G proteins, provide the important molecular basis of ligand-receptor interactions, activation and signaling mechanism, which will facilitate the next-generation drug design and the precise modulation of the endocannabinoid system. Here, we provide an overview on the structural features of cannabinoid receptors in different functional states and the diverse ligand binding modes. The major challenges and new strategies for future therapeutic applications targeting the endocannabinoid system (ECS) are also discussed.” “Cannabinoid receptors (CB1 and CB2), as part of the endocannabinoid system, play a critical role in numerous human physiological and pathological conditions. Thus, considerable efforts have been made to develop ligands for CB1 and CB2, resulting in hundreds of phyto- and synthetic cannabinoids which have shown varying affinities relevant for the treatment of various diseases. However, only a few of these ligands are clinically used.
“Cannabinoid receptors (CB1 and CB2), as part of the endocannabinoid system, play a critical role in numerous human physiological and pathological conditions. Thus, considerable efforts have been made to develop ligands for CB1 and CB2, resulting in hundreds of phyto- and synthetic cannabinoids which have shown varying affinities relevant for the treatment of various diseases. However, only a few of these ligands are clinically used. “Like most modern molecular biology and natural product chemistry, understanding cannabinoid pharmacology centers around molecular interactions, in this case, between the cannabinoids and their putative targets, the G-protein coupled receptors (GPCRs) cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2). Understanding the complex structure and interplay between the partners in this molecular dance is required to understand the mechanism of action of synthetic, endogenous, and phytochemical cannabinoids. This review, with 91 references, surveys our understanding of the structural biology of the cannabinoids and their target receptors including both a critical comparison of the extant crystal structures and the computationally derived homology models, as well as an in-depth discussion about the binding modes of the major cannabinoids. The aim is to assist in situating structural biochemists, synthetic chemists, and molecular biologists who are new to the field of cannabis research.”
“Like most modern molecular biology and natural product chemistry, understanding cannabinoid pharmacology centers around molecular interactions, in this case, between the cannabinoids and their putative targets, the G-protein coupled receptors (GPCRs) cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2). Understanding the complex structure and interplay between the partners in this molecular dance is required to understand the mechanism of action of synthetic, endogenous, and phytochemical cannabinoids. This review, with 91 references, surveys our understanding of the structural biology of the cannabinoids and their target receptors including both a critical comparison of the extant crystal structures and the computationally derived homology models, as well as an in-depth discussion about the binding modes of the major cannabinoids. The aim is to assist in situating structural biochemists, synthetic chemists, and molecular biologists who are new to the field of cannabis research.” “Background: Recent approved medicines whose active principles are Δ9Tetrahidrocannabinol (Δ9-THC) and/or cannabidiol (CBD) open novel perspectives for other phytocannabinoids also present in Cannabis sativa L. varieties. Furthermore, solid data on the potential benefits of acidic and varinic phytocannabinoids in a variety of diseases are already available. Mode of action of cannabigerol (CBG), cannabidiolic acid (CBDA), cannabigerolic acid (CBGA), cannabidivarin (CBDV) and cannabigerivarin (CBGV) is, to the very least, partial.
“Background: Recent approved medicines whose active principles are Δ9Tetrahidrocannabinol (Δ9-THC) and/or cannabidiol (CBD) open novel perspectives for other phytocannabinoids also present in Cannabis sativa L. varieties. Furthermore, solid data on the potential benefits of acidic and varinic phytocannabinoids in a variety of diseases are already available. Mode of action of cannabigerol (CBG), cannabidiolic acid (CBDA), cannabigerolic acid (CBGA), cannabidivarin (CBDV) and cannabigerivarin (CBGV) is, to the very least, partial.