“Conventional lung cancer treatments include surgery, chemotherapy and radiotherapy; however, these treatments are often poorly tolerated by patients. Cannabinoids have been studied for use as a primary cancer treatment. Cannabinoids, which are chemically similar to our own body’s endocannabinoids, can interact with signalling pathways to control the fate of cells, including cancer cells. We present a patient who declined conventional lung cancer treatment. Without the knowledge of her clinicians, she chose to self-administer ‘cannabidiol (CBD) oil’ orally 2-3 times daily. Serial imaging shows that her cancer reduced in size progressively from 41 mm to 10 mm over a period of 2.5 years. Previous studies have failed to agree on the usefulness of cannabinoids as a cancer treatment. This case appears to demonstrate a possible benefit of ‘CBD oil’ intake that may have resulted in the observed tumour regression. The use of cannabinoids as a potential cancer treatment justifies further research.”
“Conventional lung cancer treatments include surgery, chemotherapy and radiotherapy; however, these treatments are often poorly tolerated by patients. Cannabinoids have been studied for use as a primary cancer treatment. Cannabinoids, which are chemically similar to our own body’s endocannabinoids, can interact with signalling pathways to control the fate of cells, including cancer cells. We present a patient who declined conventional lung cancer treatment. Without the knowledge of her clinicians, she chose to self-administer ‘cannabidiol (CBD) oil’ orally 2-3 times daily. Serial imaging shows that her cancer reduced in size progressively from 41 mm to 10 mm over a period of 2.5 years. Previous studies have failed to agree on the usefulness of cannabinoids as a cancer treatment. This case appears to demonstrate a possible benefit of ‘CBD oil’ intake that may have resulted in the observed tumour regression. The use of cannabinoids as a potential cancer treatment justifies further research.”
https://pubmed.ncbi.nlm.nih.gov/34649854/
“Patient’s perspective
“I was not very interested in traditional cancer treatments as I was worried about the risks of surgery, and I saw my late husband suffer through the side effects of radiotherapy. My relative suggested that I should try ‘cannabidiol (CBD) oil’ to treat my cancer, and I have been taking it regularly ever since. I am ‘over the moon’ with my cancer shrinking, which I believe was caused by the ‘CBD oil’. I am tolerating it very well and I intend to take this treatment indefinitely.””
https://casereports.bmj.com/content/14/10/e244195
“Cannabis oil led to lung cancer regression in 80-year-old woman: Report”
“Case Report: Lung Cancer Shrinks in Patient Using CBD Oil”
https://www.medscape.com/viewarticle/960949

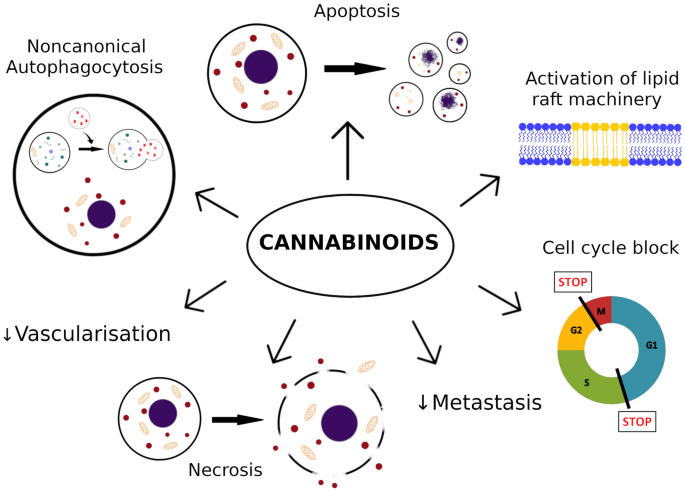
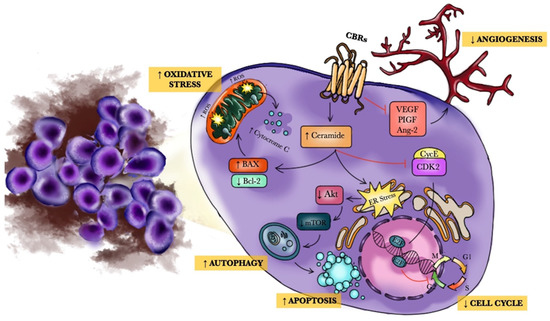
 “Cancer, as a mysterious and complex disease, has a multi-stage molecular process that uses the cellular molecular machine and multiple signaling pathways to its advantage. Cannabinoids, as terpenophenolic compounds and their derivatives, showed influences on immune system responses, inflammation, and cell growth that have sparked a growing interest in exploring their effects on cancer cell fate, as well. A large body of evidence in experimental models indicating the involvement of cannabinoids and their related receptors in cancer cell growth, development, and fate. In accordance, the present study provided insights regarding the strengths and limits of cannabinoids and their receptors in critical steps of tumorigenesis and its underlying molecular pathways such as; cancer cell proliferation, type of cell death pathway, angiogenesis, invasion, metastasis and, immune system response. Based on the results of the present study and due to the contribution of cannabinoids in various cancer cell growth control processes, these compounds cancer can be considered worthwhile in finding new alternatives for cancer therapy.”
“Cancer, as a mysterious and complex disease, has a multi-stage molecular process that uses the cellular molecular machine and multiple signaling pathways to its advantage. Cannabinoids, as terpenophenolic compounds and their derivatives, showed influences on immune system responses, inflammation, and cell growth that have sparked a growing interest in exploring their effects on cancer cell fate, as well. A large body of evidence in experimental models indicating the involvement of cannabinoids and their related receptors in cancer cell growth, development, and fate. In accordance, the present study provided insights regarding the strengths and limits of cannabinoids and their receptors in critical steps of tumorigenesis and its underlying molecular pathways such as; cancer cell proliferation, type of cell death pathway, angiogenesis, invasion, metastasis and, immune system response. Based on the results of the present study and due to the contribution of cannabinoids in various cancer cell growth control processes, these compounds cancer can be considered worthwhile in finding new alternatives for cancer therapy.” “Cannabidiol (CBD), a primary bioactive phytocannabinoid extracted from hemp, is reported to possess potent anti-tumorigenic activity in multiple cancers.
“Cannabidiol (CBD), a primary bioactive phytocannabinoid extracted from hemp, is reported to possess potent anti-tumorigenic activity in multiple cancers.  “Prostate cancer is the second most frequently occurring cancer diagnosed among males. Recent preclinical evidence implicates cannabinoids as powerful regulators of cell growth and differentiation. In this review, we focused on studies that demonstrated anticancer effects of cannabinoids and their possible mechanisms of action in prostate cancer. Besides the palliative effects of cannabinoids, research from the past two decades has demonstrated their promising potential as antitumor agents in a wide variety of cancers. This analysis may provide pharmacological insights into the selection of specific cannabinoids for the development of antitumor drugs for the treatment of prostate cancer.”
“Prostate cancer is the second most frequently occurring cancer diagnosed among males. Recent preclinical evidence implicates cannabinoids as powerful regulators of cell growth and differentiation. In this review, we focused on studies that demonstrated anticancer effects of cannabinoids and their possible mechanisms of action in prostate cancer. Besides the palliative effects of cannabinoids, research from the past two decades has demonstrated their promising potential as antitumor agents in a wide variety of cancers. This analysis may provide pharmacological insights into the selection of specific cannabinoids for the development of antitumor drugs for the treatment of prostate cancer.”
 “Cannabidiol (CBD), a phytochemical derived from
“Cannabidiol (CBD), a phytochemical derived from  “Background:
“Background:  “In humans, various sites like cannabinoid receptors (CBR) having a binding affinity with cannabinoids are distributed on the surface of different cell types, where endocannabinoids (ECs) and derivatives of fatty acid can bind. The binding of these substance(s) triggers the activation of specific receptors required for various physiological functions, including pain sensation, memory, and appetite.
“In humans, various sites like cannabinoid receptors (CBR) having a binding affinity with cannabinoids are distributed on the surface of different cell types, where endocannabinoids (ECs) and derivatives of fatty acid can bind. The binding of these substance(s) triggers the activation of specific receptors required for various physiological functions, including pain sensation, memory, and appetite. 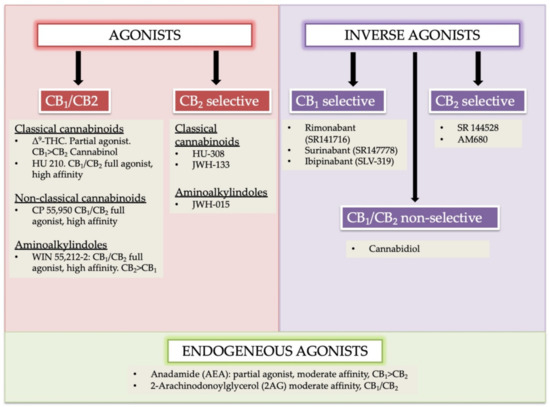
 “Melanoma is one of the most aggressive malignances in human. Recently developed therapies improved overall survival rate, however, the treatment of melanoma still remains a challenging issue.
“Melanoma is one of the most aggressive malignances in human. Recently developed therapies improved overall survival rate, however, the treatment of melanoma still remains a challenging issue.