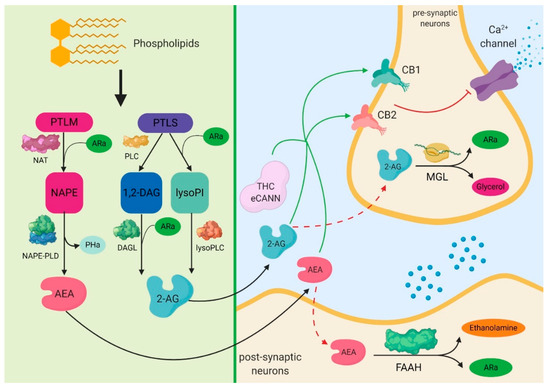
“Sepsis-induced renal damage poses a significant threat, necessitating effective therapeutic strategies. Cannabidiol (CBD) has beneficial effects on tissues and their functions by exhibiting antioxidant and anti-inflammatory effects. This study investigates the potential protective effects of CBD in mitigating lipopolysaccharide (LPS)-induced renal injury in Wistar Albino rats.
Thirty-two Wistar Albino rats were categorized into control, LPS (5 mg/kg i.p.), LPS + CBD, and CBD (5 mg/kg i.p.) groups. After the experiment, samples were collected for biochemical, genetic, histopathological, and immunohistochemical analyses. Oxidative stress markers as total oxidant status (TOS) and total antioxidant status (TAS), oxidative stress index (OSI), superoxide dismutase (SOD), glutathione peroxidase (GPx), malondialdehyde (MDA), immune staining as tumor necrosis factor alpha (TNF-α), interleukin-10 (IL-10), caspase-3, gene expressions as nuclear factor erythroid 2-related factor 2 (NRF2), C/EBP homologous protein (CHOP), caspase-9, glucose-regulating protein 78 (GRP78), B-cell leukemia/lymphoma 2 (Bcl2), and tissue histology have been examined.
The LPS-exposed group exhibited significant renal abnormalities, mitigated by CBD intervention in the LPS + CBD group. CBD reduced immunoexpression scores for TNF-α, caspase-3, and IL-10. Biochemically, CBD induced a positive shift in the oxidative balance, increasing TAS, SOD, and GPx, while decreasing TOS, OSI, and MDA levels. Genetic analyses highlighted CBD’s regulatory impact on NRF2, CHOP, caspase-9, GRP78, and Bcl2, providing molecular insights into its protective role against LPS-induced renal damage.
This study underscores CBD as a promising protective agent against sepsis-induced renal damage. Our findings could provide valuable insights into potential therapeutic avenues for addressing renal complications in sepsis.”
https://pubmed.ncbi.nlm.nih.gov/39180672/
https://link.springer.com/article/10.1007/s00210-024-03391-2







 “Natural cannabinoids may have beneficial effects on various tissues and functions including a positive influence on the immune system and the inflammatory process.
“Natural cannabinoids may have beneficial effects on various tissues and functions including a positive influence on the immune system and the inflammatory process. “Septic lung injury is one of main causes of high mortality in severe patients. Inhibition of excessive inflammatory response is considered as an effective strategy for septic lung injury.
“Septic lung injury is one of main causes of high mortality in severe patients. Inhibition of excessive inflammatory response is considered as an effective strategy for septic lung injury. “Critically ill patients with sepsis require a multidisciplinary approach, as this situation implies multiorgan distress, with most of the bodily biochemical and cellular systems being affected by the condition. Moreover, sepsis is characterized by a multitude of biochemical interactions and by dynamic changes of the immune system. At the moment, there is a gap in our understanding of the cellular, genetic, and molecular mechanisms involved in sepsis.
“Critically ill patients with sepsis require a multidisciplinary approach, as this situation implies multiorgan distress, with most of the bodily biochemical and cellular systems being affected by the condition. Moreover, sepsis is characterized by a multitude of biochemical interactions and by dynamic changes of the immune system. At the moment, there is a gap in our understanding of the cellular, genetic, and molecular mechanisms involved in sepsis.