 “Coronavirus disease (COVID-19), caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an ongoing pandemic and presents a public health emergency. It has affected millions of people and continues to affect more, despite tremendous social preventive measures. Identifying candidate drugs for the prevention and treatment of COVID-19 is crucial. The pathogenesis and the complications with advanced infection mainly involve an immune-inflammatory cascade. Therefore, therapeutic strategy relies on suppressing infectivity and inflammation, along with immune modulation.
“Coronavirus disease (COVID-19), caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an ongoing pandemic and presents a public health emergency. It has affected millions of people and continues to affect more, despite tremendous social preventive measures. Identifying candidate drugs for the prevention and treatment of COVID-19 is crucial. The pathogenesis and the complications with advanced infection mainly involve an immune-inflammatory cascade. Therefore, therapeutic strategy relies on suppressing infectivity and inflammation, along with immune modulation.
One of the most promising therapeutic targets for the modulation of immune-inflammatory responses is the endocannabinoid system, particularly the activation of cannabinoid type 2 receptors (CB2R), a G-protein coupled receptor which mediates the anti-inflammatory properties by modulating numerous signaling pathways. To pharmacologically activate the CB2 receptors, a naturally occurring cannabinoid ligand, beta-caryophyllene (BCP), received attention due to its potent anti-inflammatory, antiviral, and immunomodulatory properties. BCP is recognized as a full selective functional agonist on CB2 receptors and produces therapeutic effects by activating CB2 and the nuclear receptors, peroxisome proliferator-activated receptors (PPARs).
BCP is regarded as the first dietary cannabinoid with abundant presence across cannabis and non-cannabis plants, including spices and other edible plants. BCP showed tissue protective properties and favorably modulates numerous signaling pathways and inhibits inflammatory mediators, including cytokines, chemokines, adhesion molecules, prostanoids, and eicosanoids. Based on its pharmacological properties, molecular mechanisms, and the therapeutic potential of BCP as an immunomodulator, anti-inflammatory, organ-protective, and antiviral, we hypothesize that BCP could be a promising therapeutic and/or preventive candidate to target the triad of infection, immunity, and inflammation in COVID-19. In line with numerous studies that proposed the potential of cannabinoids in COVID-19,
BCP may be a novel candidate compound for pharmaceutical and nutraceutical development due to its unique functional receptor selectivity, wide availability and accessibility, dietary bioavailability, nonpsychoactivity, and negligible toxicity along with druggable properties, including favorable pharmacokinetic and physicochemical properties. Based on reasonable pharmacological mechanisms and therapeutic properties, we speculate that BCP has potential to be investigated against COVID-19 and will inspire further preclinical and clinical studies.”
https://pubmed.ncbi.nlm.nih.gov/34054510/
“Over the past few months, it has been suggested that modulation of the endocannabinoid system by cannabinoids, including cannabidiol, could be useful in prophylaxis and treatment of COVID-19 and may improve prognosis. Recently, extract of Cannabis sativa containing phytocannabinoids and terpenes were shown to modulate the inflammatory mediators in alveolar epithelial cells (A549) in COVID-19-associated inflammation and suggested that the phytocannabinoid mix formulation exerted better activity in comparison with individual fractions from cannabis. Many cannabinoids, including cannabidiol, have been suggested for their possible potential as preventive agents or therapeutic adjuvants with other agents in targeting the trinity of infection, inflammation, and immunity in COVID-19.”
“β-caryophyllene (BCP) is a common constitute of the essential oils of numerous spice, food plants and major component in Cannabis.” http://www.ncbi.nlm.nih.gov/pubmed/23138934
 “Objective: Joint injury-induced perturbations to the endocannabinoid system (ECS), a regulator of both inflammation and nociception, remain largely uncharacterized. We employed a mouse model of ACL rupture to assess alterations to nociception, inflammation, and the ECS while using in vitro models to determine whether CB2 agonism can mitigate inflammatory signaling in macrophages and fibroblast-like synoviocytes (FLS).
“Objective: Joint injury-induced perturbations to the endocannabinoid system (ECS), a regulator of both inflammation and nociception, remain largely uncharacterized. We employed a mouse model of ACL rupture to assess alterations to nociception, inflammation, and the ECS while using in vitro models to determine whether CB2 agonism can mitigate inflammatory signaling in macrophages and fibroblast-like synoviocytes (FLS).
 “The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems.
“The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems.  “Prostate cancer is the second most frequently occurring cancer diagnosed among males. Recent preclinical evidence implicates cannabinoids as powerful regulators of cell growth and differentiation. In this review, we focused on studies that demonstrated anticancer effects of cannabinoids and their possible mechanisms of action in prostate cancer. Besides the palliative effects of cannabinoids, research from the past two decades has demonstrated their promising potential as antitumor agents in a wide variety of cancers. This analysis may provide pharmacological insights into the selection of specific cannabinoids for the development of antitumor drugs for the treatment of prostate cancer.”
“Prostate cancer is the second most frequently occurring cancer diagnosed among males. Recent preclinical evidence implicates cannabinoids as powerful regulators of cell growth and differentiation. In this review, we focused on studies that demonstrated anticancer effects of cannabinoids and their possible mechanisms of action in prostate cancer. Besides the palliative effects of cannabinoids, research from the past two decades has demonstrated their promising potential as antitumor agents in a wide variety of cancers. This analysis may provide pharmacological insights into the selection of specific cannabinoids for the development of antitumor drugs for the treatment of prostate cancer.”
 “Colic is a common digestive disorder in horses and one of the most urgent problems in equine medicine. A growing body of literature has indicated that the activation of cannabinoid receptors could exert beneficial effects on gastrointestinal inflammation and visceral hypersensitivity.
“Colic is a common digestive disorder in horses and one of the most urgent problems in equine medicine. A growing body of literature has indicated that the activation of cannabinoid receptors could exert beneficial effects on gastrointestinal inflammation and visceral hypersensitivity.  “Background:
“Background: 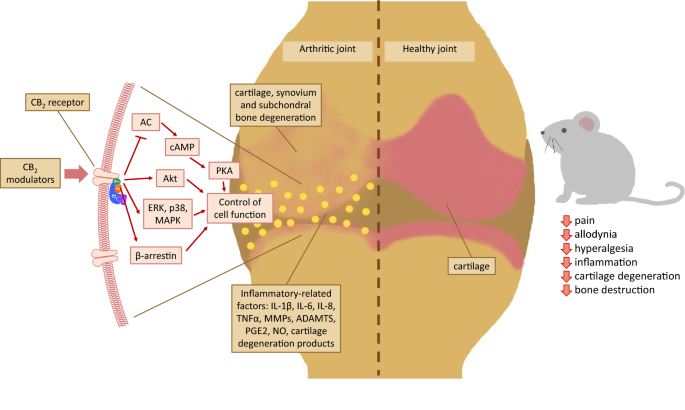 “Over the last several decades, the percentage of patients suffering from different forms of arthritis has increased due to the ageing population and the increasing risk of civilization diseases, e.g. obesity, which contributes to arthritis development. Osteoarthritis and rheumatoid arthritis are estimated to affect 50-60% of people over 65 years old and cause serious health and economic problems. Currently, therapeutic strategies are limited and focus mainly on pain attenuation and maintaining joint functionality. First-line therapies are nonsteroidal anti-inflammatory drugs; in more advanced stages, stronger analgesics, such as opioids, are required, and in the most severe cases, joint arthroplasty is the only option to ensure joint mobility.
“Over the last several decades, the percentage of patients suffering from different forms of arthritis has increased due to the ageing population and the increasing risk of civilization diseases, e.g. obesity, which contributes to arthritis development. Osteoarthritis and rheumatoid arthritis are estimated to affect 50-60% of people over 65 years old and cause serious health and economic problems. Currently, therapeutic strategies are limited and focus mainly on pain attenuation and maintaining joint functionality. First-line therapies are nonsteroidal anti-inflammatory drugs; in more advanced stages, stronger analgesics, such as opioids, are required, and in the most severe cases, joint arthroplasty is the only option to ensure joint mobility.  “Coronavirus disease (COVID-19), caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an ongoing pandemic and presents a public health emergency. It has affected millions of people and continues to affect more, despite tremendous social preventive measures. Identifying candidate drugs for the prevention and treatment of COVID-19 is crucial. The pathogenesis and the complications with advanced infection mainly involve an immune-inflammatory cascade. Therefore, therapeutic strategy relies on suppressing infectivity and inflammation, along with immune modulation.
“Coronavirus disease (COVID-19), caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an ongoing pandemic and presents a public health emergency. It has affected millions of people and continues to affect more, despite tremendous social preventive measures. Identifying candidate drugs for the prevention and treatment of COVID-19 is crucial. The pathogenesis and the complications with advanced infection mainly involve an immune-inflammatory cascade. Therefore, therapeutic strategy relies on suppressing infectivity and inflammation, along with immune modulation.  “Colorectal cancer (CRC) is between the top three occurring cancers worldwide. The anticancer effects of Cannabinoid receptor 2 (CB
“Colorectal cancer (CRC) is between the top three occurring cancers worldwide. The anticancer effects of Cannabinoid receptor 2 (CB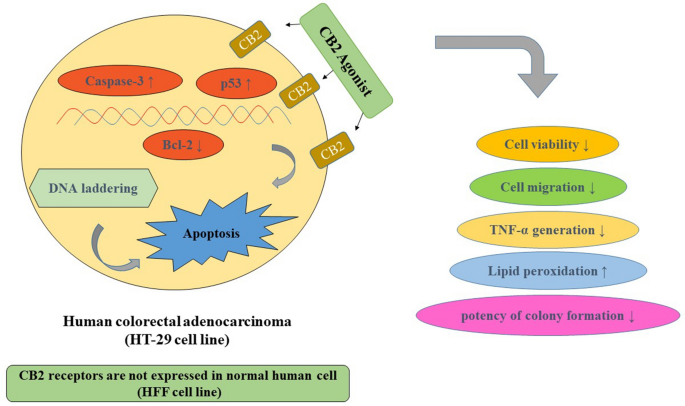
 “Alzheimer’s disease (AD) is characterized by structural damage, death, and functional disruption of cholinergic neurons (ChNs) as a result of intracellular amyloid-β (Aβ) aggregation, extracellular neuritic plaques, and hyperphosphorylation of protein tau (p-Tau) overtime.
“Alzheimer’s disease (AD) is characterized by structural damage, death, and functional disruption of cholinergic neurons (ChNs) as a result of intracellular amyloid-β (Aβ) aggregation, extracellular neuritic plaques, and hyperphosphorylation of protein tau (p-Tau) overtime.