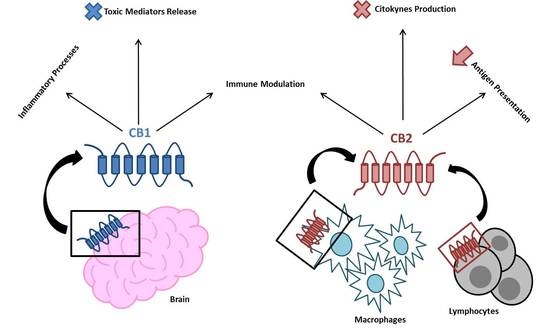
“The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems.
“The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems.
In addition to regulating physiological processes, the ECS directly influences anxiety, feeding behaviour/appetite, emotional behaviour, depression, nervous functions, neurogenesis, neuroprotection, reward, cognition, learning, memory, pain sensation, fertility, pregnancy, and pre-and post-natal development.
The ECS is also involved in several pathophysiological diseases such as cancer, cardiovascular diseases, and neurodegenerative diseases. In recent years, genetic and pharmacological manipulation of the ECS has gained significant interest in medicine, research, and drug discovery and development.
The distribution of the components of the ECS system throughout the body, and the physiological/pathophysiological role of the ECS-signalling pathways in many diseases, all offer promising opportunities for the development of novel cannabinergic, cannabimimetic, and cannabinoid-based therapeutic drugs that genetically or pharmacologically modulate the ECS via inhibition of metabolic pathways and/or agonism or antagonism of the receptors of the ECS. This modulation results in the differential expression/activity of the components of the ECS that may be beneficial in the treatment of a number of diseases.
This manuscript in-depth review will investigate the potential of the ECS in the treatment of various diseases, and to put forth the suggestion that many of these secondary metabolites of Cannabis sativa L. (hereafter referred to as “C. sativa L.” or “medical cannabis”), may also have potential as lead compounds in the development of cannabinoid-based pharmaceuticals for a variety of diseases.”
https://www.mdpi.com/1422-0067/22/17/9472

 “Endocannabinoid system consists of
“Endocannabinoid system consists of