 “Aging is a complex phenomenon associated with a wide spectrum of physical and physiological changes affecting every part of all metazoans, if they escape death prior to reaching maturity. Critical to survival, the immune system evolved as the principal component of response to injury and defense against pathogen invasions. Because how significantly immune system affects and is affected by aging, several neologisms now appear to encapsulate these reciprocal relationships, such as Immunosenescence. The central part of Immunosenescence is Inflammaging -a sustained, low-grade, sterile inflammation occurring after reaching reproductive prime. Once initiated, the impact of Inflammaging and its adverse effects determine the direction and magnitudes of further Inflammaging. In this article, we review the nature of this vicious cycle, we will propose that phytocannabinoids as immune regulators may possess the potential as effective adjunctive therapies to slow and, in certain cases, reverse the pathologic senescence to permit a more healthy aging.”
“Aging is a complex phenomenon associated with a wide spectrum of physical and physiological changes affecting every part of all metazoans, if they escape death prior to reaching maturity. Critical to survival, the immune system evolved as the principal component of response to injury and defense against pathogen invasions. Because how significantly immune system affects and is affected by aging, several neologisms now appear to encapsulate these reciprocal relationships, such as Immunosenescence. The central part of Immunosenescence is Inflammaging -a sustained, low-grade, sterile inflammation occurring after reaching reproductive prime. Once initiated, the impact of Inflammaging and its adverse effects determine the direction and magnitudes of further Inflammaging. In this article, we review the nature of this vicious cycle, we will propose that phytocannabinoids as immune regulators may possess the potential as effective adjunctive therapies to slow and, in certain cases, reverse the pathologic senescence to permit a more healthy aging.”
“The beneficial effects of cannabinoids may be considered as alternative therapy in treating age-related diseases.”
https://www.sciencedirect.com/science/article/pii/S1568163721002348?via%3Dihub

 “Colic is a common digestive disorder in horses and one of the most urgent problems in equine medicine. A growing body of literature has indicated that the activation of cannabinoid receptors could exert beneficial effects on gastrointestinal inflammation and visceral hypersensitivity.
“Colic is a common digestive disorder in horses and one of the most urgent problems in equine medicine. A growing body of literature has indicated that the activation of cannabinoid receptors could exert beneficial effects on gastrointestinal inflammation and visceral hypersensitivity.  “Ehlers-Danlos Syndromes (EDS) and related Hypermobility Spectrum Disorders (HSD) are debilitating connective tissue disorders that feature a prominent pain component for which there are limited therapeutic options for pain management.
“Ehlers-Danlos Syndromes (EDS) and related Hypermobility Spectrum Disorders (HSD) are debilitating connective tissue disorders that feature a prominent pain component for which there are limited therapeutic options for pain management.  “Cannabis sativa
“Cannabis sativa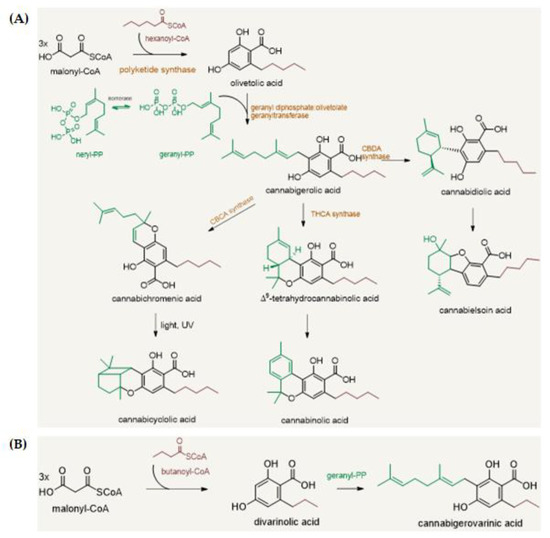
 “Venous Leg Ulcers are highly prevalent lower limb integumentary wounds that remain challenging to heal despite the use of evidence-based compression therapies. A multitude of adjuvant treatments have been studied but none have demonstrated enough efficacy to gain adoption into treatment guidelines.
“Venous Leg Ulcers are highly prevalent lower limb integumentary wounds that remain challenging to heal despite the use of evidence-based compression therapies. A multitude of adjuvant treatments have been studied but none have demonstrated enough efficacy to gain adoption into treatment guidelines.  “Agents targeting the endocannabinoid system (ECS) have gained attention as potential cancer treatments. Given recent evidence that cannabinoid receptor 2 (CB2R) regulates lymphocyte development and inflammation, we performed studies on CB2R in the immune response against melanoma. Analysis of The Cancer Genome Atlas (TCGA) data revealed a strong positive correlation between CB2R expression and survival, as well as B cell infiltration in human melanoma. In a murine melanoma model, CB2R expression reduced the growth of melanoma as well as the B cell frequencies in the tumor microenvironment (TME), compared to CB2R-deficient mice. In depth analysis of tumor-infiltrating B cells using single-cell RNA sequencing suggested a less differentiated phenotype in tumors from
“Agents targeting the endocannabinoid system (ECS) have gained attention as potential cancer treatments. Given recent evidence that cannabinoid receptor 2 (CB2R) regulates lymphocyte development and inflammation, we performed studies on CB2R in the immune response against melanoma. Analysis of The Cancer Genome Atlas (TCGA) data revealed a strong positive correlation between CB2R expression and survival, as well as B cell infiltration in human melanoma. In a murine melanoma model, CB2R expression reduced the growth of melanoma as well as the B cell frequencies in the tumor microenvironment (TME), compared to CB2R-deficient mice. In depth analysis of tumor-infiltrating B cells using single-cell RNA sequencing suggested a less differentiated phenotype in tumors from 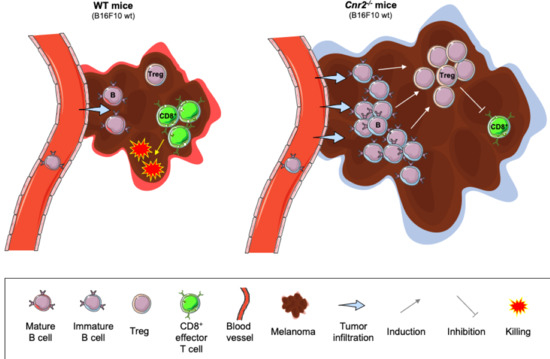
 “In recent years, evidence has accumulated that cannabinoids-especially the non-psychoactive compound, cannabidiol (CBD)-possess promising medical and pharmacological activities that might qualify them as potential anti-tumor drugs. This review is based on multiple studies summarizing different mechanisms for how CBD can target tumor cells including cannabinoid receptors or other constituents of the endocannabinoid system, and their complex activation of biological systems that results in the inhibition of tumor growth. CBD also participates in anti-inflammatory activities which are related to tumor progression, as demonstrated in preclinical models. Although the numbers of clinical trials and tested tumor entities are limited, there is clear evidence that CBD has anti-tumor efficacy and is well tolerated in human cancer patients. In summary, it appears that CBD has potential as a neoadjuvant and/or adjuvant drug in therapy for cancer.”
“In recent years, evidence has accumulated that cannabinoids-especially the non-psychoactive compound, cannabidiol (CBD)-possess promising medical and pharmacological activities that might qualify them as potential anti-tumor drugs. This review is based on multiple studies summarizing different mechanisms for how CBD can target tumor cells including cannabinoid receptors or other constituents of the endocannabinoid system, and their complex activation of biological systems that results in the inhibition of tumor growth. CBD also participates in anti-inflammatory activities which are related to tumor progression, as demonstrated in preclinical models. Although the numbers of clinical trials and tested tumor entities are limited, there is clear evidence that CBD has anti-tumor efficacy and is well tolerated in human cancer patients. In summary, it appears that CBD has potential as a neoadjuvant and/or adjuvant drug in therapy for cancer.” “We previously reported that cannabidiol (CBD), a cannabinoid with a low toxicity profile, downregulated the expression of the prometastatic gene inhibitor of DNA binding 1 (
“We previously reported that cannabidiol (CBD), a cannabinoid with a low toxicity profile, downregulated the expression of the prometastatic gene inhibitor of DNA binding 1 ( “Glioblastoma is the most aggressive cancer among primary brain tumours. As with other cancers, the incidence of glioblastoma is increasing; despite modern therapies, the overall mean survival of patients post-diagnosis averages around 16 months, a figure that has not changed in many years. Cannabigerol (CBG) has only recently been reported to prevent the progression of certain carcinomas and has not yet been studied in glioblastoma. Here, we have compared the cytotoxic, apoptotic, and anti-invasive effects of the purified natural cannabinoid CBG together with CBD and THC on established differentiated glioblastoma tumour cells and glioblastoma stem cells. CBG and THC reduced the viability of both types of cells to a similar extent, whereas combining CBD with CBG was more efficient than with THC. CBD and CBG, both alone and in combination, induced caspase-dependent cell apoptosis, and there was no additive THC effect. Of note, CBG inhibited glioblastoma invasion in a similar manner to CBD and the chemotherapeutic temozolomide. We have demonstrated that THC has little added value in combined-cannabinoid glioblastoma treatment, suggesting that this psychotropic cannabinoid should be replaced with CBG in future clinical studies of glioblastoma therapy.”
“Glioblastoma is the most aggressive cancer among primary brain tumours. As with other cancers, the incidence of glioblastoma is increasing; despite modern therapies, the overall mean survival of patients post-diagnosis averages around 16 months, a figure that has not changed in many years. Cannabigerol (CBG) has only recently been reported to prevent the progression of certain carcinomas and has not yet been studied in glioblastoma. Here, we have compared the cytotoxic, apoptotic, and anti-invasive effects of the purified natural cannabinoid CBG together with CBD and THC on established differentiated glioblastoma tumour cells and glioblastoma stem cells. CBG and THC reduced the viability of both types of cells to a similar extent, whereas combining CBD with CBG was more efficient than with THC. CBD and CBG, both alone and in combination, induced caspase-dependent cell apoptosis, and there was no additive THC effect. Of note, CBG inhibited glioblastoma invasion in a similar manner to CBD and the chemotherapeutic temozolomide. We have demonstrated that THC has little added value in combined-cannabinoid glioblastoma treatment, suggesting that this psychotropic cannabinoid should be replaced with CBG in future clinical studies of glioblastoma therapy.” “Plant-based therapies date back centuries. Cannabis sativa is one such plant that was used medicinally up until the early part of the 20th century.
“Plant-based therapies date back centuries. Cannabis sativa is one such plant that was used medicinally up until the early part of the 20th century.