“The isolation and identification of the discrete plant cannabinoids in marijuana revived interest in analyzing historical therapeutic claims made for cannabis in clinical case studies and anecdotes. In particular, sources as old as the 11th and 15th centuries claimed efficacy for crude marijuana extracts in the treatment of convulsive disorders, prompting a particularly active area of preclinical research into the therapeutic potential of plant cannabinoids in epilepsy.
Since that time, a large body of literature has accumulated describing the effects of several of the >100 individual plant cannabinoids in preclinical models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. We surveyed the literature for relevant reports of such plant cannabinoid effects and critically reviewed their findings.
We found that acute CB1R agonism in simple models of acute seizures in rodents typically produces anti-convulsant effects whereas CB1R antagonists exert converse effects in the same models. However, when the effects of such ligands are examined in more complex models of epilepsy, epileptogenesis and neuroprotection, a less simplistic narrative emerges.
Here, the complex interactions between (i) brain regions involved in a given model, (ii) relative contributions of endocannabinoid signaling to modulation of synaptic transmission in such areas, (iii) multi-target effects, (iv) cannabinoid type 1 and type 2 receptor signaling interactions and, (v) timing, (vi) duration and (vii) localization of ligand administration suggest that there is both anti-epileptic therapeutic potential and a pro-epileptic risk in up- and down-regulation of endocannabinoid signaling in the central nervous system.
Factors such receptor desensitization and specific pharmacology of ligands used (e.g. full vs partial agonists and neutral antagonists vs inverse agonists) also appear to play an important role in the effects reported.
Furthermore, the effects of several plant cannabinoids, most notably cannabidiol (CBD) and cannabidavarin (CBDV), in models of seizures, epilepsy, epileptogenesis, and neuroprotection are less ambiguous, and consistent with reports of therapeutically beneficial effects of these compounds in clinical studies.
However, continued paucity of firm information regarding the therapeutic molecular mechanism of CBD/CBDV highlights the continued need for research in this area in order to identify as yet under-exploited targets for drug development and raise our understanding of treatment-resistant epilepsies.
The recent reporting of positive results for cannabidiol treatment in two Phase III clinical trials in treatment-resistant epilepsies provides pivotal evidence of clinical efficacy for one plant cannabinoid in epilepsy.
Moreover, risks and/or benefits associated with the use of unlicensed Δ9-THC containing marijuana extracts in pediatric epilepsies remain poorly understood.
Therefore, in light of these paradigm-changing clinical events, the present review’s findings aim to drive future drug development for newly-identified targets and indications, identify important limitations of animal models in the investigation of plant cannabinoid effects in the epilepsies, and focuses future research in this area on specific, unanswered questions regarding the complexities of endocannabinoid signaling in epilepsy.”
https://www.ncbi.nlm.nih.gov/pubmed/28190698
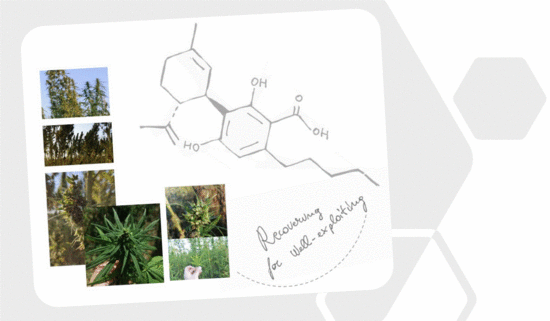
 “Cannabidiolic acid (CBDA) is the main phytocannabinoid in fiber and seed-oil hemp (Cannabis sativa L.) plants, but its potential health-related capabilities have been masked for years by a greater scientific interest towards its neutral derivative cannabidiol (CBD). This review aims to collect from the literature and critically discuss all the information about this molecule, starting from its biosynthesis, and focusing on its bioactivity, as an anti-inflammatory, anti-emetic, anti-convulsant, and anti-cancerogenic drug. Furthermore, in the awareness that, despite its multiple bioactive effects, currently poor efforts have been made to achieve its reliable purification, herein, we propose a relatively simple, fast, and inexpensive procedure for its recovery from pollen of industrial hemp cultivars. Spectroscopic and spectrometric techniques allowed us to unequivocally identify pure isolated CBDA and to distinguish it from the constitutional isomer tetrahydrocannabinolic acid (THCA-A).”
“Cannabidiolic acid (CBDA) is the main phytocannabinoid in fiber and seed-oil hemp (Cannabis sativa L.) plants, but its potential health-related capabilities have been masked for years by a greater scientific interest towards its neutral derivative cannabidiol (CBD). This review aims to collect from the literature and critically discuss all the information about this molecule, starting from its biosynthesis, and focusing on its bioactivity, as an anti-inflammatory, anti-emetic, anti-convulsant, and anti-cancerogenic drug. Furthermore, in the awareness that, despite its multiple bioactive effects, currently poor efforts have been made to achieve its reliable purification, herein, we propose a relatively simple, fast, and inexpensive procedure for its recovery from pollen of industrial hemp cultivars. Spectroscopic and spectrometric techniques allowed us to unequivocally identify pure isolated CBDA and to distinguish it from the constitutional isomer tetrahydrocannabinolic acid (THCA-A).”