 “Positive effect of some cannabinoids in the treatment and prophylaxis of a wide variety of oxidation-associated diseases and growing popularity of supplements containing cannabinoids, mainly cannabinoid oils (e.g. CBD oil, CBG oil), in the self-medication of humans cause a growing interest in the antioxidant properties of these compounds, especially those not showing psychotropic effects.
“Positive effect of some cannabinoids in the treatment and prophylaxis of a wide variety of oxidation-associated diseases and growing popularity of supplements containing cannabinoids, mainly cannabinoid oils (e.g. CBD oil, CBG oil), in the self-medication of humans cause a growing interest in the antioxidant properties of these compounds, especially those not showing psychotropic effects.
Herein, we report the antioxidant activity of cannabigerol (CBG), cannabidiol (CBD), Δ9-tetrahydrocannabinol (Δ9-THC), cannabinol (CBN), cannabigerolic acid (CBGA), cannabinolic acid (CBDA) and Δ9-tetrahydrocannabinolic acid (Δ9-THCA) estimated by spectrophotometric methods: ABTS, DPPH, ORAC, beta-carotene CUPRAC and FRAP.
The presented data prove that all the examined cannabinoids exhibit antioxidant activity manifested in their ability to scavenge free radicals, to prevent the oxidation process and to reduce metal ions. Although the intensity of these activities is not the same for the individual cannabinoids it is comparable for all of them with that of E vitamin.”
“The present paper discusses the antioxidant properties of CBG, CBN, CBDA, CBGA and Δ9-THCA which, beside CBD and Δ9-THC, are also supposed to be bioactive compounds useful in the therapeutic treatment of different diseases. According to the literature, CBD and Δ9-THC exhibit strong antioxidant activity, stronger than vitamins C, A and E.
The presented data prove that all the examined cannabinoids – CBG, CBD, Δ9-THC, CBN, CBGA CBDA and Δ9-THCA – exhibit antioxidant activity manifesting itself in their ability to scavenge free radicals, to protect oxidation process and to reduce metal ions. Although, the intensity of these activities for individual cannabinoids is not the same, it is generally comparable to that of E vitamin.” https://www.sciencedirect.com/science/article/pii/S0367326X21000903?via%3Dihub

 “Salicylic acid (SA) is a plant hormone which plays a crucial role in the plant defense against various pathogens and abiotic stresses. Increasing reports suggest that this phenolic compound and its derivatives, collectively termed salicylates, not only regulate plant defense but also have beneficial effects on human health. Both natural and synthetic salicylates are known to have multiple targets in humans, thereby exhibiting various appreciating pharmacological roles, including anti-inflammatory, anticancer, neuroprotective, antidiabetic effects, and so on. The role of some salicylates, such as acetylsalicylic acid (aspirin), 5-aminosalicylic acid (mesalazine), and amorfrutins in human diseases has been well studied in vitro. However, their clinical significance in different diseases is largely unknown. Based on recent studies, five natural salicylates, including amorfrutin, ginkgolic acid, grifolic acid, tetrahydrocannabinolic acid, and cannabidiolic acid, showed potential roles in different challenging human diseases. This review summarizes together some of the recent information on multitarget regulatory activities of these natural salicylates and their pharmacological roles in human health.”
“Salicylic acid (SA) is a plant hormone which plays a crucial role in the plant defense against various pathogens and abiotic stresses. Increasing reports suggest that this phenolic compound and its derivatives, collectively termed salicylates, not only regulate plant defense but also have beneficial effects on human health. Both natural and synthetic salicylates are known to have multiple targets in humans, thereby exhibiting various appreciating pharmacological roles, including anti-inflammatory, anticancer, neuroprotective, antidiabetic effects, and so on. The role of some salicylates, such as acetylsalicylic acid (aspirin), 5-aminosalicylic acid (mesalazine), and amorfrutins in human diseases has been well studied in vitro. However, their clinical significance in different diseases is largely unknown. Based on recent studies, five natural salicylates, including amorfrutin, ginkgolic acid, grifolic acid, tetrahydrocannabinolic acid, and cannabidiolic acid, showed potential roles in different challenging human diseases. This review summarizes together some of the recent information on multitarget regulatory activities of these natural salicylates and their pharmacological roles in human health.”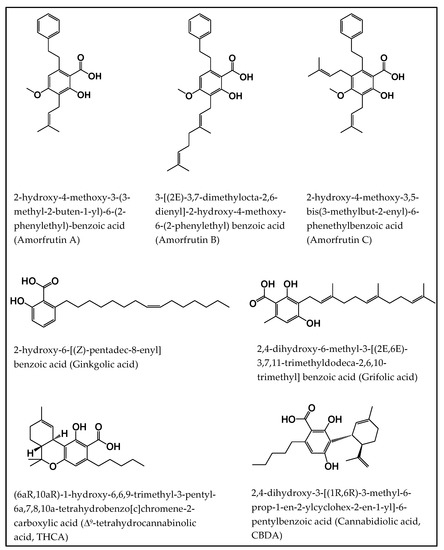
 “Low tetrahydrocannabinol Cannabis sativa products, also known as hemp products, have become widely available and their use in veterinary patients has become increasingly popular. Despite prevalence of use, the veterinary literature is lacking and evidence-based resource for cannabinoid efficacy.
“Low tetrahydrocannabinol Cannabis sativa products, also known as hemp products, have become widely available and their use in veterinary patients has become increasingly popular. Despite prevalence of use, the veterinary literature is lacking and evidence-based resource for cannabinoid efficacy. “Rett syndrome (RTT) is a rare neurologic disorder, characterized by severe behavioural and physiological symptoms. RTT is caused by mutations in the MECP2 gene in about 95% of cases and to date no cure is available.
“Rett syndrome (RTT) is a rare neurologic disorder, characterized by severe behavioural and physiological symptoms. RTT is caused by mutations in the MECP2 gene in about 95% of cases and to date no cure is available. “The emergence of multi-drug resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) causes a major threat to public health due to its limited therapeutic options.
“The emergence of multi-drug resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) causes a major threat to public health due to its limited therapeutic options.
 “Cannabidiolic acid (CBDA) is the main phytocannabinoid in fiber and seed-oil hemp (Cannabis sativa L.) plants, but its potential health-related capabilities have been masked for years by a greater scientific interest towards its neutral derivative cannabidiol (CBD). This review aims to collect from the literature and critically discuss all the information about this molecule, starting from its biosynthesis, and focusing on its bioactivity, as an anti-inflammatory, anti-emetic, anti-convulsant, and anti-cancerogenic drug. Furthermore, in the awareness that, despite its multiple bioactive effects, currently poor efforts have been made to achieve its reliable purification, herein, we propose a relatively simple, fast, and inexpensive procedure for its recovery from pollen of industrial hemp cultivars. Spectroscopic and spectrometric techniques allowed us to unequivocally identify pure isolated CBDA and to distinguish it from the constitutional isomer tetrahydrocannabinolic acid (THCA-A).”
“Cannabidiolic acid (CBDA) is the main phytocannabinoid in fiber and seed-oil hemp (Cannabis sativa L.) plants, but its potential health-related capabilities have been masked for years by a greater scientific interest towards its neutral derivative cannabidiol (CBD). This review aims to collect from the literature and critically discuss all the information about this molecule, starting from its biosynthesis, and focusing on its bioactivity, as an anti-inflammatory, anti-emetic, anti-convulsant, and anti-cancerogenic drug. Furthermore, in the awareness that, despite its multiple bioactive effects, currently poor efforts have been made to achieve its reliable purification, herein, we propose a relatively simple, fast, and inexpensive procedure for its recovery from pollen of industrial hemp cultivars. Spectroscopic and spectrometric techniques allowed us to unequivocally identify pure isolated CBDA and to distinguish it from the constitutional isomer tetrahydrocannabinolic acid (THCA-A).”
 “Rationale: When acutely administered intraperitoneally, the non-psychoactive cannabinoid cannabidiol (CBD), its acidic precursor cannabidiolic acid (CBDA) and a stable methyl ester of CBDA (HU-580) reduce lithium chloride (LiCl)-induced conditioned gaping in male rats (a selective preclinical model of acute nausea) via activation of the serotonin 1A (5-HT1A) receptor.
“Rationale: When acutely administered intraperitoneally, the non-psychoactive cannabinoid cannabidiol (CBD), its acidic precursor cannabidiolic acid (CBDA) and a stable methyl ester of CBDA (HU-580) reduce lithium chloride (LiCl)-induced conditioned gaping in male rats (a selective preclinical model of acute nausea) via activation of the serotonin 1A (5-HT1A) receptor. “The major phytocannabinoid cannabidiol (
“The major phytocannabinoid cannabidiol ( “The cellular microenvironment plays a critical role in the maintenance of bone marrow-derived mesenchymal stem cells (BM-MSCs) and their subsequent cell lineage differentiation. Recent studies suggested that individuals with adipocyte-related metabolic disorders have altered function and adipogenic potential of adipose stem cell subpopulations, primarily BM-MSCs, increasing the risk of heart attack, stroke or diabetes.
“The cellular microenvironment plays a critical role in the maintenance of bone marrow-derived mesenchymal stem cells (BM-MSCs) and their subsequent cell lineage differentiation. Recent studies suggested that individuals with adipocyte-related metabolic disorders have altered function and adipogenic potential of adipose stem cell subpopulations, primarily BM-MSCs, increasing the risk of heart attack, stroke or diabetes.