 “Cannabinoids are a group of terpenophenolic compounds derived from the Cannabis sativa L. plant. There is a growing body of evidence from cell culture and animal studies in support of cannabinoids possessing anticancer properties.
“Cannabinoids are a group of terpenophenolic compounds derived from the Cannabis sativa L. plant. There is a growing body of evidence from cell culture and animal studies in support of cannabinoids possessing anticancer properties.
Method: A database search of peer reviewed articles published in English as full texts between January 1970 and April 2021 in Google Scholar, MEDLINE, PubMed and Web of Science was undertaken. References of relevant literature were searched to identify additional studies to construct a narrative literature review of oncological effects of cannabinoids in pre-clinical and clinical studies in various cancer types.
Results: Phyto-, endogenous and synthetic cannabinoids demonstrated antitumour effects both in vitro and in vivo. However, these effects are dependent on cancer type, the concentration and preparation of the cannabinoid and the abundance of receptor targets. The mechanism of action of synthetic cannabinoids, (-)-trans-Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) has mainly been described via the traditional cannabinoid receptors; CB1 and CB2, but reports have also indicated evidence of activity through GPR55, TRPM8 and other ion channels including TRPA1, TRPV1 and TRPV2.
Conclusion: Cannabinoids have shown to be efficacious both as a single agent and in combination with antineoplastic drugs. These effects have occurred through various receptors and ligands and modulation of signalling pathways involved in hallmarks of cancer pathology. There is a need for further studies to characterise its mode of action at the molecular level and to delineate efficacious dosage and route of administration in addition to synergistic regimes.”
https://pubmed.ncbi.nlm.nih.gov/34259916/
“Since time immemorial, the Cannabis plant has been used as a source of fibre, herbal remedy, medicinal and religious purposes. In the mid-nineteenth century, O’Shaughnessy and Moreau reported positive effects of cannabis on muscle spasms, vomiting, convulsions, rheumatism, tetanus, and rabies. However, during the twentieth century, its utilisation in Western medicine started to decline as a result of political prejudices and economic interests rather than scientific or medical reasons.
Plant-based, endogenous and synthetic cannabinoid compounds have shown merits in not only alleviating the unwanted side effects of antineoplastic drug regiments, but have also shown promising evidence in decreasing tumour burden, and one in vivo study so far concludes increasing survival rates in mice. Various extracted forms of cannabinoids from C. sativa have shown varying cytotoxic effects which should be explored in more detail in future studies as majority of the evidence originates from studies investigating mainly ∆9-THC and CBD’s actions.”
https://link.springer.com/article/10.1007/s00432-021-03710-7

 “As the major nonpsychotropic constituent of
“As the major nonpsychotropic constituent of 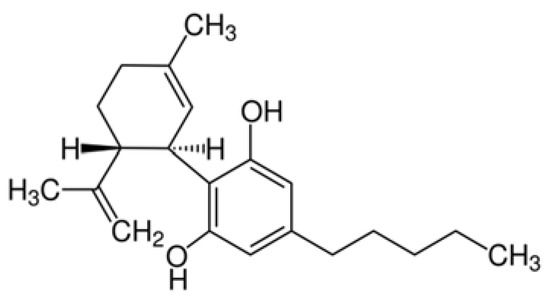

 “In this work, we evaluated, for the first time, the antitumor effect of cannabidiol (CBD) as monotherapy and in combination with conventional chemotherapeutics in ovarian cancer and developed PLGA-microparticles as CBD carriers to optimize its anticancer activity.
“In this work, we evaluated, for the first time, the antitumor effect of cannabidiol (CBD) as monotherapy and in combination with conventional chemotherapeutics in ovarian cancer and developed PLGA-microparticles as CBD carriers to optimize its anticancer activity.




