“Our main aim was to investigate the short‐term therapeutic effects, safety/tolerability and potential side effects of the cannabis galenical preparation (Bedrocan) in patients with a range of chronic conditions unresponsive to other treatments.
“Our main aim was to investigate the short‐term therapeutic effects, safety/tolerability and potential side effects of the cannabis galenical preparation (Bedrocan) in patients with a range of chronic conditions unresponsive to other treatments.
These data suggest that a cannabis galenical preparation may be therapeutically effective and safe for the symptomatic treatment of some chronic diseases.
The findings suggested that patients affected by chronic long‐standing (months or years) advanced disease, who had not responded to standard treatment, had improved symptoms when they were treated with Bedrocan. The galenical treatment contributed not only to decreased pain but also to restored physical function in this cohort after 3 months and improvement in overall QOL.”

 “Agitation is a prevalent and difficult-to-treat symptom in patients with moderate-to-severe Alzheimer’s disease (AD). Though there are nonpharmacological and pharmacological interventions recommended for the treatment of agitation, the efficacy of these are modest and not always consistent. Furthermore, the safety profiles of currently prescribed medications are questionable.
“Agitation is a prevalent and difficult-to-treat symptom in patients with moderate-to-severe Alzheimer’s disease (AD). Though there are nonpharmacological and pharmacological interventions recommended for the treatment of agitation, the efficacy of these are modest and not always consistent. Furthermore, the safety profiles of currently prescribed medications are questionable. “Rett syndrome (RTT) is an X-linked neurodevelopmental disorder caused mainly by mutations in the MECP2 gene, being one of the leading causes of mental disability in females.
“Rett syndrome (RTT) is an X-linked neurodevelopmental disorder caused mainly by mutations in the MECP2 gene, being one of the leading causes of mental disability in females. “Recent evidence has raised in discussion the possibility that
“Recent evidence has raised in discussion the possibility that  “There is uncertainty regarding the appropriate dose of
“There is uncertainty regarding the appropriate dose of  “The Cannabis plant has been used for many of years as a medicinal agent in the relief of pain and seizures. It contains approximately 540 natural compounds including more than 100 that have been identified as phytocannabinoids due to their shared chemical structure. The predominant psychotropic component is Δ9-tetrahydrocannabinol (Δ9-THC), while the major non-psychoactive ingredient is
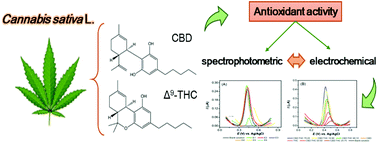
“The Cannabis plant has been used for many of years as a medicinal agent in the relief of pain and seizures. It contains approximately 540 natural compounds including more than 100 that have been identified as phytocannabinoids due to their shared chemical structure. The predominant psychotropic component is Δ9-tetrahydrocannabinol (Δ9-THC), while the major non-psychoactive ingredient is  “Delta-9-tetrahydrocannabinol (THC) is the primary psychoactive compound in
“Delta-9-tetrahydrocannabinol (THC) is the primary psychoactive compound in 
 “Herein, we report the antioxidant activity of
“Herein, we report the antioxidant activity of