 “Pancreatic cancer (PC) is related to lifestyle risks, chronic inflammation, and germline mutations in BRCA1/2, ATM, MLH1, TP53, or CDKN2A. Surgical resection and adjuvant chemotherapy are the main therapeutic strategies but are less effective in patients with high-grade tumors.
“Pancreatic cancer (PC) is related to lifestyle risks, chronic inflammation, and germline mutations in BRCA1/2, ATM, MLH1, TP53, or CDKN2A. Surgical resection and adjuvant chemotherapy are the main therapeutic strategies but are less effective in patients with high-grade tumors.
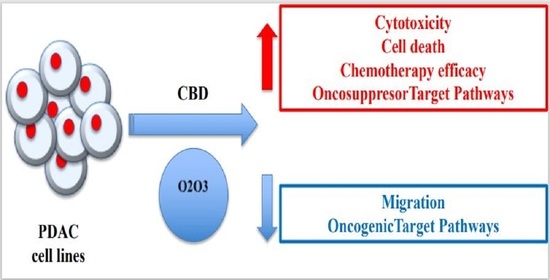
Oxygen-ozone (O2/O3) therapy is an emerging alternative tool for the treatment of several clinical disorders. O2/O3 therapy has been found to ameliorate mechanisms promoting chronic pain and inflammation, including hypoxia, inflammatory mediators, and infection.
The advantages of using cannabinoids have been evaluated in vitro and in vivo models of several human cancers. Regarding PDAC, activation of cannabinoid receptors was found to induce pancreatic cancer cell apoptosis without affecting the normal pancreas cells.
In a murine model of PDAC, a combination of cannabidiol (CBD) and gemcitabine increased survival length by nearly three times. Herein, we evaluate the anticancer effect of CBD and O2/O3, alone or in combination, on two human PDAC cell lines, PANC-1 and MiaPaCa-2, examining expression profiles of 92 pancreatic adenocarcinoma associated genes, cytotoxicity, migration properties, and cell death. Finally, we assess the combination effects with gemcitabine and paclitaxel.
Summarizing, for the first time the antitumoral effect of combined therapy with CBD and oxygen-ozone therapy in PDAC is evidenced.”
https://pubmed.ncbi.nlm.nih.gov/32992648/
https://www.mdpi.com/2072-6694/12/10/2774

 “Cannabinoids are increasingly-used substances in the treatment of chronic pain, some neuropsychiatric disorders and more recently, skin disorders with an inflammatory component.
“Cannabinoids are increasingly-used substances in the treatment of chronic pain, some neuropsychiatric disorders and more recently, skin disorders with an inflammatory component.
 “Glioblastoma multiforme (GBM) is the most frequent and aggressive type of brain tumor due, at least in part, to its poor response to current anticancer treatments. These features could be explained, at least partially, by the presence within the tumor mass of a small population of cells termed Glioma Initiating Cells (GICs) that has been proposed to be responsible for the relapses occurring in this disease. Thus, the development of novel therapeutic approaches (and specifically those targeting the population of GICs) is urgently needed to improve the survival of the patients suffering this devastating disease.
Previous observations by our group and others have shown that Δ9-Tetrahydrocannabinol (THC, the main active ingredient of marijuana) and other cannabinoids including
“Glioblastoma multiforme (GBM) is the most frequent and aggressive type of brain tumor due, at least in part, to its poor response to current anticancer treatments. These features could be explained, at least partially, by the presence within the tumor mass of a small population of cells termed Glioma Initiating Cells (GICs) that has been proposed to be responsible for the relapses occurring in this disease. Thus, the development of novel therapeutic approaches (and specifically those targeting the population of GICs) is urgently needed to improve the survival of the patients suffering this devastating disease.
Previous observations by our group and others have shown that Δ9-Tetrahydrocannabinol (THC, the main active ingredient of marijuana) and other cannabinoids including  “Glioblastoma (GBM) is the most common and aggressive brain tumor, which causes the highest number of deaths worldwide. It is a highly vascularized tumor, infiltrative, and its tumorigenic capacity is exacerbated. All these hallmarks are therapeutic targets in GBM treatment, including surgical removal followed by radiotherapy and chemotherapy.
Current therapies have not been sufficient for the effective patient’s management, so the classic therapies have had to expand and incorporate new alternative treatments, including natural compounds.
This review summarizes natural products and their physiological effects in in vitro and in vivo models of GBM, specifically by modulating signaling pathways involved in angiogenesis, cell migration/invasion, cell viability, apoptosis, and chemoresistance. The most important aspects of natural products and their derivatives were described in relation to its antitumoral effects.
As a final result, it can be obtained that within the compounds with more evidence that supports or suggests its clinical use are the
“Glioblastoma (GBM) is the most common and aggressive brain tumor, which causes the highest number of deaths worldwide. It is a highly vascularized tumor, infiltrative, and its tumorigenic capacity is exacerbated. All these hallmarks are therapeutic targets in GBM treatment, including surgical removal followed by radiotherapy and chemotherapy.
Current therapies have not been sufficient for the effective patient’s management, so the classic therapies have had to expand and incorporate new alternative treatments, including natural compounds.
This review summarizes natural products and their physiological effects in in vitro and in vivo models of GBM, specifically by modulating signaling pathways involved in angiogenesis, cell migration/invasion, cell viability, apoptosis, and chemoresistance. The most important aspects of natural products and their derivatives were described in relation to its antitumoral effects.
As a final result, it can be obtained that within the compounds with more evidence that supports or suggests its clinical use are the 
 “The endocannabinoid system is currently under intense investigation due to the therapeutic potential of
“The endocannabinoid system is currently under intense investigation due to the therapeutic potential of  “In different models of paralytic ileus, cannabinoid receptors are overexpressed and endogenous cannabinoids are massively released, contributing to gastrointestinal dysmotility. The antitumoral drug vincristine depresses gastrointestinal motility and a similar mechanism could participate in this effect. Therefore, our aim was to determine, using CB1 and CB2 antagonists, whether an increased endocannabinoid tone is involved in vincristine-induced gastrointestinal ileus.
Key results: Vincristine induced damage to the mucosa of ileum and colon and reduced gastrointestinal motor function at 0.5 mg/kg. The effect on motor function was particularly evident when the study started 24 h after administration. AM251, but not AM630, significantly prevented vincristine effect, particularly in the small intestine, when administered thrice. AM251 alone did not significantly alter gastrointestinal motility.
Conclusions: The fact that AM251, but not AM630, is capable of reducing the effect of vincristine suggests that, like in other experimental models of paralytic ileus, an increased cannabinoid tone develops and is at least partially responsible for the alterations induced by the antitumoral drug on gastrointestinal motor function. Thus, CB1 antagonists might be useful to prevent/treat ileus induced by vincristine.”
“In different models of paralytic ileus, cannabinoid receptors are overexpressed and endogenous cannabinoids are massively released, contributing to gastrointestinal dysmotility. The antitumoral drug vincristine depresses gastrointestinal motility and a similar mechanism could participate in this effect. Therefore, our aim was to determine, using CB1 and CB2 antagonists, whether an increased endocannabinoid tone is involved in vincristine-induced gastrointestinal ileus.
Key results: Vincristine induced damage to the mucosa of ileum and colon and reduced gastrointestinal motor function at 0.5 mg/kg. The effect on motor function was particularly evident when the study started 24 h after administration. AM251, but not AM630, significantly prevented vincristine effect, particularly in the small intestine, when administered thrice. AM251 alone did not significantly alter gastrointestinal motility.
Conclusions: The fact that AM251, but not AM630, is capable of reducing the effect of vincristine suggests that, like in other experimental models of paralytic ileus, an increased cannabinoid tone develops and is at least partially responsible for the alterations induced by the antitumoral drug on gastrointestinal motor function. Thus, CB1 antagonists might be useful to prevent/treat ileus induced by vincristine.”