“Cannabis sativa and its principal components, Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol, are increasingly being used to treat a variety of medical problems, including inflammatory conditions.
“Cannabis sativa and its principal components, Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol, are increasingly being used to treat a variety of medical problems, including inflammatory conditions.
Although studies suggest that the endocannabinoid system has immunomodulatory properties, there remains a paucity of information on the effects of cannabinoids on immunity and on outcomes of infection and injury.
We investigated the effects and mechanism(s) of action of cannabinoid receptor agonists, including Δ9-THC, on inflammation and organ injury in endotoxemic mice.
Administration of Δ9-THC caused a dramatic early upregulation of plasma IL-10 levels, reduced plasma IL-6 and CCL-2 levels, led to better clinical status, and attenuated organ injury in endotoxemic mice. The anti-inflammatory effects of Δ9-THC in endotoxemic mice were reversed by a cannabinoid receptor type 1 (CB1R) inverse agonist (SR141716), and by clodronate-induced myeloid-cell depletion, but not by genetic invalidation or blockade of other putative Δ9-THC receptors, including cannabinoid receptor type 2, TRPV1, GPR18, GPR55, and GPR119. Although Δ9-THC administration reduced the activation of several spleen immune cell subsets, the anti-inflammatory effects of Δ9-THC were preserved in splenectomized endotoxemic mice. Finally, using IL-10-GFP reporter mice, we showed that blood monocytic myeloid-derived suppressive cells mediate the Δ9-THC-induced early rise in circulating IL-10.
These results indicate that Δ9-THC potently induces IL-10, while reducing proinflammatory cytokines, chemokines, and related organ injury in endotoxemic mice via the activation of CB1R. These data have implications for acute and chronic conditions that are driven by dysregulated inflammation, such as sepsis, and raise the possibility that CB1R-signaling may constitute a novel target for inflammatory disorders.”
https://www.ncbi.nlm.nih.gov/pubmed/32385136
https://www.jimmunol.org/content/early/2020/05/07/jimmunol.2000213
 “Growing evidence recognises cannabinoid receptors as potential therapeutic targets for pain. Consequently, there is increasing interest in developing cannabinoid receptor agonists for treating pain.
“Growing evidence recognises cannabinoid receptors as potential therapeutic targets for pain. Consequently, there is increasing interest in developing cannabinoid receptor agonists for treating pain.
 “Obesity rates are increasing worldwide and there is a need for novel therapeutic treatment options.
“Obesity rates are increasing worldwide and there is a need for novel therapeutic treatment options. “Δ9‐THCA‐A, the precursor of Δ9‐THC, is a non‐psychotropic phytocannabinoid that shows PPARγ agonistic activity. Herein, we investigated Δ9‐THCA ability to modulate classic cannabinoid receptors (CB1 and CB2) and evaluated its anti‐arthritis activity.
“Δ9‐THCA‐A, the precursor of Δ9‐THC, is a non‐psychotropic phytocannabinoid that shows PPARγ agonistic activity. Herein, we investigated Δ9‐THCA ability to modulate classic cannabinoid receptors (CB1 and CB2) and evaluated its anti‐arthritis activity.
 “Multiple myeloma (MM) is characterized by aberrant bone marrow plasma cell (PC) proliferation and is one of the most common hematological malignancies. The potential effect of cannabinoids on the immune system and hematological malignancies has been poorly characterized.
“Multiple myeloma (MM) is characterized by aberrant bone marrow plasma cell (PC) proliferation and is one of the most common hematological malignancies. The potential effect of cannabinoids on the immune system and hematological malignancies has been poorly characterized.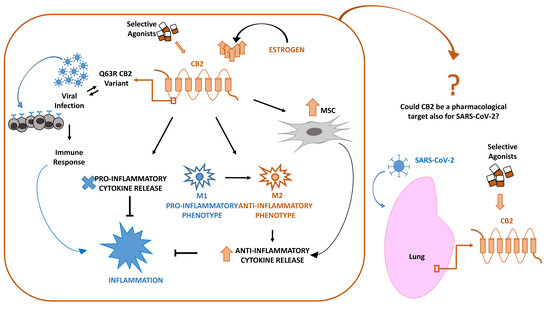
 “Historical relevance: Cannabis sativa L. (C. sativa) is a plant whose use as a therapeutic agent shares its origins with the first Far East’s human societies. Cannabis has been used not only for recreational purposes, but as a food to obtain textile fibers, to produce hemp paper, to treat many physical and mental disorders.
“Historical relevance: Cannabis sativa L. (C. sativa) is a plant whose use as a therapeutic agent shares its origins with the first Far East’s human societies. Cannabis has been used not only for recreational purposes, but as a food to obtain textile fibers, to produce hemp paper, to treat many physical and mental disorders. “HIV infection affects an estimated 38 million people. Approximately 50% of HIV patients exhibit neurocognitive dysfunction termed HIV-Associated Neurocognitive Disorder (HAND). HAND is a consequence of chronic low-level neuroinflammation due to HIV entry into the brain. Initially, monocytes become activated in circulation and traffic to the brain. Monocytes, when activated, become susceptible to infection by HIV and can then carry the virus across the blood brain barrier. Once in the brain, activated monocytes secrete chemokines, which recruit virus-specific CD8+ T cells into the brain to further promote neuroinflammation. HAND is closely linked to systemic inflammation driven, in part, by HIV but is also due to persistent translocation of microorganisms across the GI tract. Persistent anti-viral responses in the GI tract compromise microbial barrier integrity. Indeed, HIV patients can exhibit remarkably high levels of activated (CD16+) monocytes in circulation.
“HIV infection affects an estimated 38 million people. Approximately 50% of HIV patients exhibit neurocognitive dysfunction termed HIV-Associated Neurocognitive Disorder (HAND). HAND is a consequence of chronic low-level neuroinflammation due to HIV entry into the brain. Initially, monocytes become activated in circulation and traffic to the brain. Monocytes, when activated, become susceptible to infection by HIV and can then carry the virus across the blood brain barrier. Once in the brain, activated monocytes secrete chemokines, which recruit virus-specific CD8+ T cells into the brain to further promote neuroinflammation. HAND is closely linked to systemic inflammation driven, in part, by HIV but is also due to persistent translocation of microorganisms across the GI tract. Persistent anti-viral responses in the GI tract compromise microbial barrier integrity. Indeed, HIV patients can exhibit remarkably high levels of activated (CD16+) monocytes in circulation.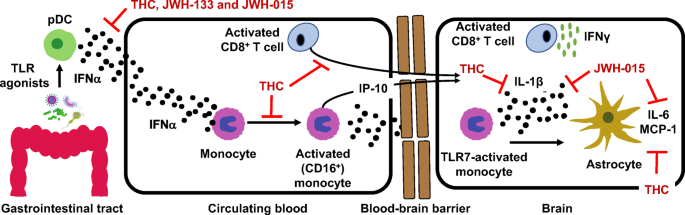
 “Cannabis sativa and its principal components, Δ9-tetrahydrocannabinol (Δ9-THC) and
“Cannabis sativa and its principal components, Δ9-tetrahydrocannabinol (Δ9-THC) and