“Can Medical Cannabis Help to Cure SCD?”
“Sickle cell disease (SCD) is a hereditary condition caused by a mutation in the haemoglobin gene, which leads to symptoms of anaemia, extreme pain, and organ damage if unmanaged.”
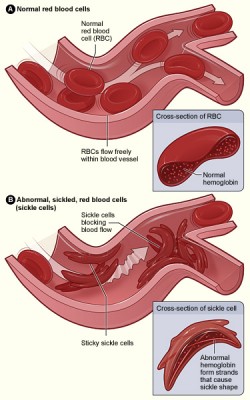
“Individuals suffering from SCD are far more likely to use cannabis than the general population, potentially for its analgesic properties.
In 2010, researchers at the University of Minnesota found that the synthetic THC analogue CP 55,940 was as effective as morphine sulphate in treating SCD-related severe pain in transgenic mice expressing human sickle haemoglobin, and that it was effective at smaller doses than the opioid.
In 2011, a further paper submitted by the same researchers to Blood (the Journal of the American Association of Hematology) indicated that CP 55,940 ameliorated severe pain associated with the hypoxia/reoxygenation cycle. CP 55,940 is a full agonist of both CB receptors, and is thought to act as an antagonist at the GPR55 receptor.
As well as this, cannabis has been repeatedly shown to act as a vasodilator, which could in itself assist in easing the blockages caused by build-up of sickle cells…
SCD is a painful and debilitating disease, and the overall inefficacy of opioid treatments and resultant poor quality of life for many sufferers is an indication that our approach to it is far from perfect.
If cannabis is a good candidate to replace opioids, it should be implemented forthwith to prevent ongoing suffering for existing patients.”
http://sensiseeds.com/en/blog/sickle-cell-pain-may-managed-cannabis/




