 “Opioid misuse and overuse has contributed to a widespread overdose crisis and many patients and physicians are considering medical cannabis to support opioid tapering and chronic pain control. Using a five-step modified Delphi process, we aimed to develop consensus-based recommendations on: 1) when and how to safely initiate and titrate cannabinoids in the presence of opioids, 2) when and how to safely taper opioids in the presence of cannabinoids, and 3) how to monitor patients and evaluate outcomes when treating with opioids and cannabinoids.
“Opioid misuse and overuse has contributed to a widespread overdose crisis and many patients and physicians are considering medical cannabis to support opioid tapering and chronic pain control. Using a five-step modified Delphi process, we aimed to develop consensus-based recommendations on: 1) when and how to safely initiate and titrate cannabinoids in the presence of opioids, 2) when and how to safely taper opioids in the presence of cannabinoids, and 3) how to monitor patients and evaluate outcomes when treating with opioids and cannabinoids.
Results: In patients with chronic pain taking opioids not reaching treatment goals, there was consensus that cannabinoids may be considered for patients experiencing or displaying opioid-related complications, despite psychological or physical interventions. There was consensus observed to initiate with a cannabidiol (CBD)-predominant oral extract in the daytime and consider adding tetrahydrocannabinol (THC). When adding THC, start with 0.5-3 mg, and increase by 1-2 mg once or twice weekly up to 30-40 mg/day. Initiate opioid tapering when the patient reports a minor/major improvement in function, seeks less as-needed medication to control pain, and/or the cannabis dose has been optimized. The opioid tapering schedule may be 5%-10% of the morphine equivalent dose (MED) every 1 to 4 weeks. Clinical success could be defined by an improvement in function/quality of life, a ≥ 30% reduction in pain intensity, a ≥ 25% reduction in opioid dose, a reduction in opioid dose to < 90 mg MED, and/or reduction in opioid-related adverse events.
Conclusions: This five-stage modified Delphi process led to the development of consensus-based recommendations surrounding the safe introduction and titration of cannabinoids in concert with tapering opioids.”

 “Low tetrahydrocannabinol Cannabis sativa products, also known as hemp products, have become widely available and their use in veterinary patients has become increasingly popular. Despite prevalence of use, the veterinary literature is lacking and evidence-based resource for cannabinoid efficacy.
“Low tetrahydrocannabinol Cannabis sativa products, also known as hemp products, have become widely available and their use in veterinary patients has become increasingly popular. Despite prevalence of use, the veterinary literature is lacking and evidence-based resource for cannabinoid efficacy. “Cannabis has long been used for healing and recreation in several regions of the world. Over 400 bioactive constituents, including more than 100 phytocannabinoids, have been isolated from this plant. The non-psychoactive cannabidiol (CBD) and the psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) are the major and widely studied constituents from this plant.
“Cannabis has long been used for healing and recreation in several regions of the world. Over 400 bioactive constituents, including more than 100 phytocannabinoids, have been isolated from this plant. The non-psychoactive cannabidiol (CBD) and the psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) are the major and widely studied constituents from this plant. “Coronavirus disease 2019 (COVID-19) is a highly infectious respiratory disease caused by the severe acute respiratory syndrome coronavirus 2. A significant proportion of COVID-19 patients develop Acute Respiratory Distress Syndrome (ARDS) resulting from hyperactivation of the immune system and cytokine storm, which leads to respiratory and multi-organ failure, and death. Currently, there are no effective treatments against hyperimmune syndrome and ARDS.
“Coronavirus disease 2019 (COVID-19) is a highly infectious respiratory disease caused by the severe acute respiratory syndrome coronavirus 2. A significant proportion of COVID-19 patients develop Acute Respiratory Distress Syndrome (ARDS) resulting from hyperactivation of the immune system and cytokine storm, which leads to respiratory and multi-organ failure, and death. Currently, there are no effective treatments against hyperimmune syndrome and ARDS. “Cannabidiol (CBD) has anti-tumorigenic activity. However, the anti-cancer effect of CBD on head and neck squamous cell carcinoma (HNSCC) remains unclear. The cytotoxicity of CBD on HNSCC was analyzed using cell survival and colony-forming assays in vitro.
“Cannabidiol (CBD) has anti-tumorigenic activity. However, the anti-cancer effect of CBD on head and neck squamous cell carcinoma (HNSCC) remains unclear. The cytotoxicity of CBD on HNSCC was analyzed using cell survival and colony-forming assays in vitro. “Research within a gynecologic oncology population has lagged behind the uptake in use of medical cannabis for symptom control. This study seeks to evaluate patient experience with prescribed medical cannabis obtained through licensed dispensaries in women with gynecologic malignancies.
“Research within a gynecologic oncology population has lagged behind the uptake in use of medical cannabis for symptom control. This study seeks to evaluate patient experience with prescribed medical cannabis obtained through licensed dispensaries in women with gynecologic malignancies. “The potential therapeutic use of some Cannabis sativa plant compounds has been attracting great interest, especially for managing neuropsychiatric disorders due to the relative lack of efficacy of the current treatments.
“The potential therapeutic use of some Cannabis sativa plant compounds has been attracting great interest, especially for managing neuropsychiatric disorders due to the relative lack of efficacy of the current treatments.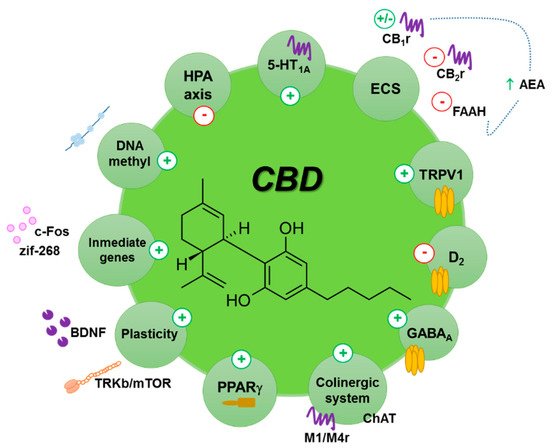
 “Little is known about the patients’ view on treatment with medical cannabis (MC) for Parkinson’s disease (PD).
“Little is known about the patients’ view on treatment with medical cannabis (MC) for Parkinson’s disease (PD). “With the current COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), there is an urgent need for new therapies and prevention strategies that can help curtail disease spread and reduce mortality.
“With the current COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), there is an urgent need for new therapies and prevention strategies that can help curtail disease spread and reduce mortality.