 “It is well known that certain active ingredients of the plants of Cannabis genus, i.e., the “phytocannabinoids” [pCBs; e.g., (−)-trans-Δ9-tetrahydrocannabinol (THC), (−)-cannabidiol, etc.] can influence a wide array of biological processes, and the human body is able to produce endogenous analogs of these substances [“endocannabinoids” (eCB), e.g., arachidonoylethanolamine (anandamide, AEA), 2-arachidonoylglycerol (2-AG), etc.]. These ligands, together with multiple receptors (e.g., CB1 and CB2 cannabinoid receptors, etc.), and a complex enzyme and transporter apparatus involved in the synthesis and degradation of the ligands constitute the endocannabinoid system (ECS), a recently emerging regulator of several physiological processes. The ECS is widely expressed in the human body, including several members of the innate and adaptive immune system, where eCBs, as well as several pCBs were shown to deeply influence immune functions thereby regulating inflammation, autoimmunity, antitumor, as well as antipathogen immune responses, etc. Based on this knowledge, many in vitro and in vivo studies aimed at exploiting the putative therapeutic potential of cannabinoid signaling in inflammation-accompanied diseases (e.g., multiple sclerosis) or in organ transplantation, and to dissect the complex immunological effects of medical and “recreational” marijuana consumption. Thus, the objective of the current article is (i) to summarize the most recent findings of the field; (ii) to highlight the putative therapeutic potential of targeting cannabinoid signaling; (iii) to identify open questions and key challenges; and (iv) to suggest promising future directions for cannabinoid-based drug development.
“It is well known that certain active ingredients of the plants of Cannabis genus, i.e., the “phytocannabinoids” [pCBs; e.g., (−)-trans-Δ9-tetrahydrocannabinol (THC), (−)-cannabidiol, etc.] can influence a wide array of biological processes, and the human body is able to produce endogenous analogs of these substances [“endocannabinoids” (eCB), e.g., arachidonoylethanolamine (anandamide, AEA), 2-arachidonoylglycerol (2-AG), etc.]. These ligands, together with multiple receptors (e.g., CB1 and CB2 cannabinoid receptors, etc.), and a complex enzyme and transporter apparatus involved in the synthesis and degradation of the ligands constitute the endocannabinoid system (ECS), a recently emerging regulator of several physiological processes. The ECS is widely expressed in the human body, including several members of the innate and adaptive immune system, where eCBs, as well as several pCBs were shown to deeply influence immune functions thereby regulating inflammation, autoimmunity, antitumor, as well as antipathogen immune responses, etc. Based on this knowledge, many in vitro and in vivo studies aimed at exploiting the putative therapeutic potential of cannabinoid signaling in inflammation-accompanied diseases (e.g., multiple sclerosis) or in organ transplantation, and to dissect the complex immunological effects of medical and “recreational” marijuana consumption. Thus, the objective of the current article is (i) to summarize the most recent findings of the field; (ii) to highlight the putative therapeutic potential of targeting cannabinoid signaling; (iii) to identify open questions and key challenges; and (iv) to suggest promising future directions for cannabinoid-based drug development.
Active Components of Cannabis sativa (Hemp)—Phytocannabinoids (pCBs) and Beyond
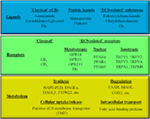
It is known since ancient times that consumption of different parts of the plant Cannabis sativa can lead to psychotropic effects. Moreover, mostly, but not exclusively because of its potent analgesic actions, it was considered to be beneficial in the management of several diseases. Nowadays it is a common knowledge that these effects were mediated by the complex mixture of biologically active substances produced by the plant. So far, at least 545 active compounds have been identified in it, among which, the best-studied ones are the so-called pCBs. It is also noteworthy that besides these compounds, ca. 140 different terpenes [including the potent and selective CB2 agonist sesquiterpene β-caryophyllene (BCP)], multiple flavonoids, alkanes, sugars, non-cannabinoid phenols, phenylpropanoids, steroids, fatty acids, and various nitrogenous compounds can be found in the plant, individual biological actions of which are mostly still nebulous. Among the so far identified > 100 pCBs, the psychotropic (−)-trans-Δ9-tetrahydrocannabinol (THC) and the non-psychotropic (−)-cannabidiol (CBD) are the best-studied ones, exerting a wide-variety of biological actions [including but not exclusively: anticonvulsive, analgesic, antiemetic, and anti inflammatory effects]. Of great importance, pCBs have been shown to modulate the activity of a plethora of cellular targets, extending their impact far beyond the “classical” (see above) cannabinoid signaling. Indeed, besides being agonists [or in some cases even antagonists of CB1 and CB2 cannabinoid receptors, some pCBs were shown to differentially modulate the activity of certain TRP channels, PPARs, serotonin, α adrenergic, adenosine or opioid receptors, and to inhibit COX and lipoxygenase enzymes, FAAH, EMT, etc.. Moreover, from a clinical point-of-view, it should also be noted that pCBs can indirectly modify pharmacokinetics of multiple drugs (e.g., cyclosporine A) by interacting with several cytochrome P 450 (CYP) enzymes. Taken together, pCBs can be considered as multitarget polypharmacons, each of them having unique “molecular fingerprints” created by the characteristic activation/inhibition pattern of its locally available cellular targets.
Concluding Remarks—Lessons to Learn from Cannabis
Research efforts of the past few decades have unambiguously evidenced that ECS is one of the central orchestrators of both innate and adaptive immune systems, and that pure pCBs as well as complex cannabis-derivatives can also deeply influence immune responses. Although, many open questions await to be answered, pharmacological modulation of the (endo)cannabinoid signaling, and restoration of the homeostatic eCB tone of the tissues augur to be very promising future directions in the management of several pathological inflammation-accompanied diseases. Moreover, in depth analysis of the (quite complex) mechanism-of-action of the most promising pCBs is likely to shed light to previously unknown immune regulatory mechanisms and can therefore pave new “high”-ways toward developing completely novel classes of therapeutic agents to manage a wide-variety of diseases.”
https://www.frontiersin.org/articles/10.3389/fimmu.2017.01487/full
 “Nuclear factor erythroid 2-related factor 2 (NRF2) is a pleiotropic transcription factor that has neuroprotective and anti-inflammatory effects, regulating more than 250 genes. As NRF2, cannabinoid receptor type 2 (CB2) is also implicated in the preservation of neurons against glia-driven inflammation. To this concern, little is known about the regulation pathways implicated in CB2 receptor expression. In this study, we analyze whether NRF2 could modulate the transcription of CB2 in neuronal and microglial cells. Bioinformatics analysis revealed an antioxidant response element in the promoter sequence of the CB2 receptor gene. Further analysis by chemical and genetic manipulations of this transcription factor demonstrated that NRF2 is not able to modulate the expression of CB2 in neurons. On the other hand, at the level of microglia, the expression of CB2 is NRF2-dependent. These results are related to the differential levels of expression of both genes regarding the brain cell type. Since modulation of CB2 receptor signaling may represent a promising therapeutic target with minimal psychotropic effects that can be used to modulate endocannabinoid-based therapeutic approaches and to reduce neurodegeneration, our findings will contribute to disclose the potential of CB2 as a novel target for treating different pathologies.”
“Nuclear factor erythroid 2-related factor 2 (NRF2) is a pleiotropic transcription factor that has neuroprotective and anti-inflammatory effects, regulating more than 250 genes. As NRF2, cannabinoid receptor type 2 (CB2) is also implicated in the preservation of neurons against glia-driven inflammation. To this concern, little is known about the regulation pathways implicated in CB2 receptor expression. In this study, we analyze whether NRF2 could modulate the transcription of CB2 in neuronal and microglial cells. Bioinformatics analysis revealed an antioxidant response element in the promoter sequence of the CB2 receptor gene. Further analysis by chemical and genetic manipulations of this transcription factor demonstrated that NRF2 is not able to modulate the expression of CB2 in neurons. On the other hand, at the level of microglia, the expression of CB2 is NRF2-dependent. These results are related to the differential levels of expression of both genes regarding the brain cell type. Since modulation of CB2 receptor signaling may represent a promising therapeutic target with minimal psychotropic effects that can be used to modulate endocannabinoid-based therapeutic approaches and to reduce neurodegeneration, our findings will contribute to disclose the potential of CB2 as a novel target for treating different pathologies.”
 “Δ9-tetrahydrocannabinol (Δ9-THC), the primary active component in Cannabis sativa preparations such as hashish and marijuana, signals by binding to cell surface receptors. Two types of receptors have been cloned and characterized as cannabinoid (CB) receptors. CB1 receptors (CB1R) are ubiquitously present in the central nervous
“Δ9-tetrahydrocannabinol (Δ9-THC), the primary active component in Cannabis sativa preparations such as hashish and marijuana, signals by binding to cell surface receptors. Two types of receptors have been cloned and characterized as cannabinoid (CB) receptors. CB1 receptors (CB1R) are ubiquitously present in the central nervous  “Combat veterans are at elevated suicide risk. The goal of this study was to test the hypothesis that combat veterans who have made a suicide attempt post-deployment can be distinguished from combat veterans who have never made a suicide attempt based on differences in psychological and biological variables. For the latter, we focused on endogenous cannabinoids, neuroendocrine markers that are associated with stress. Demographic and clinical parameters of suicide attempters and non-attempters were assessed. Blood samples were assayed for anandamide (AEA), 2-arachidonoylglycerol (2-AG), and cortisol. Suicide attempters had higher Scale for Suicidal Ideation (SSI) scores in comparison to non-attempters. Controlling for gender, 2-AG levels were higher among suicide attempters in comparison to non-attempters. Cortisol levels positively correlated with 2-AG levels and negatively correlated with SSI scores among non-attempters but not among attempters. AEA levels negatively correlated with SSI scores among attempters but not among non-attempters. Our results indicate that there are psychological and biological differences between combat veterans with or without a history of suicidal attempt. Our findings also suggest that clinically observed differences between the groups may have a neurobiological basis.”
“Combat veterans are at elevated suicide risk. The goal of this study was to test the hypothesis that combat veterans who have made a suicide attempt post-deployment can be distinguished from combat veterans who have never made a suicide attempt based on differences in psychological and biological variables. For the latter, we focused on endogenous cannabinoids, neuroendocrine markers that are associated with stress. Demographic and clinical parameters of suicide attempters and non-attempters were assessed. Blood samples were assayed for anandamide (AEA), 2-arachidonoylglycerol (2-AG), and cortisol. Suicide attempters had higher Scale for Suicidal Ideation (SSI) scores in comparison to non-attempters. Controlling for gender, 2-AG levels were higher among suicide attempters in comparison to non-attempters. Cortisol levels positively correlated with 2-AG levels and negatively correlated with SSI scores among non-attempters but not among attempters. AEA levels negatively correlated with SSI scores among attempters but not among non-attempters. Our results indicate that there are psychological and biological differences between combat veterans with or without a history of suicidal attempt. Our findings also suggest that clinically observed differences between the groups may have a neurobiological basis.” “The G-protein coupled cannabinoid receptor 2 (CB2) has been implicated in the regulation of adult neurogenesis in the hippocampus. The contribution of CB2 towards basal levels of proliferation and the number of neural progenitors in the subgranular zone (SGZ) of the dentate gyrus, however, remain unclear. We stained hippocampal brain sections of 16- to 17-week-old wildtype and CB2-deficient mice, for neural progenitor and immature neuron markers doublecortin (DCX) and calretinin (CR) and for the proliferation marker Ki67 and quantified the number of positive cells in the SGZ. The quantification revealed that CB2 deficiency neither altered overall cell proliferation nor the size of the DCX+ or DCX and CR double-positive populations in the SGZ compared to control animals. The results indicate that CB2 might not contribute to basal levels of adult neurogenesis in four-month-old healthy mice. CB2 signaling might be more relevant in conditions where adult neurogenesis is dynamically regulated, such as neuroinflammation.”
“The G-protein coupled cannabinoid receptor 2 (CB2) has been implicated in the regulation of adult neurogenesis in the hippocampus. The contribution of CB2 towards basal levels of proliferation and the number of neural progenitors in the subgranular zone (SGZ) of the dentate gyrus, however, remain unclear. We stained hippocampal brain sections of 16- to 17-week-old wildtype and CB2-deficient mice, for neural progenitor and immature neuron markers doublecortin (DCX) and calretinin (CR) and for the proliferation marker Ki67 and quantified the number of positive cells in the SGZ. The quantification revealed that CB2 deficiency neither altered overall cell proliferation nor the size of the DCX+ or DCX and CR double-positive populations in the SGZ compared to control animals. The results indicate that CB2 might not contribute to basal levels of adult neurogenesis in four-month-old healthy mice. CB2 signaling might be more relevant in conditions where adult neurogenesis is dynamically regulated, such as neuroinflammation.” “High rates of relapse are a chronic and debilitating obstacle to effective treatment of alcohol use disorder (AUD); however, no effective treatments are available to treat symptoms induced by protracted abstinence.
“High rates of relapse are a chronic and debilitating obstacle to effective treatment of alcohol use disorder (AUD); however, no effective treatments are available to treat symptoms induced by protracted abstinence. “Ischemia and reperfusion of intestinal tissue (intestinal I/R) induces disruption of ileal contractility and chain responses of inflammatory.
“Ischemia and reperfusion of intestinal tissue (intestinal I/R) induces disruption of ileal contractility and chain responses of inflammatory. “Aging is an inevitable process that involves changes along life in multiple neurochemical, neuroanatomical, hormonal systems, and many others. In addition, these biological modifications lead to an increase in age-related sickness such as cardiovascular diseases, osteoporosis, neurodegenerative disorders, and sleep disturbances, among others that affect activities of daily life. Demographic projections have demonstrated that aging will increase its worldwide rate in the coming years. The research on chronic diseases of the elderly is important to gain insights into this growing global burden.
“Aging is an inevitable process that involves changes along life in multiple neurochemical, neuroanatomical, hormonal systems, and many others. In addition, these biological modifications lead to an increase in age-related sickness such as cardiovascular diseases, osteoporosis, neurodegenerative disorders, and sleep disturbances, among others that affect activities of daily life. Demographic projections have demonstrated that aging will increase its worldwide rate in the coming years. The research on chronic diseases of the elderly is important to gain insights into this growing global burden. “Endocannabinoids (ECs) are important regulators of cell signaling.
“Endocannabinoids (ECs) are important regulators of cell signaling. “As the prevalence of kidney disease continues to rise worldwide, there is accumulating evidence that kidney injury and dysfunction, whether acute or chronic, is associated with major adverse outcomes, including mortality. Meanwhile, effective therapeutic options in the treatment of acute kidney injury (AKI) and chronic kidney disease (CKD) have been sparse.
“As the prevalence of kidney disease continues to rise worldwide, there is accumulating evidence that kidney injury and dysfunction, whether acute or chronic, is associated with major adverse outcomes, including mortality. Meanwhile, effective therapeutic options in the treatment of acute kidney injury (AKI) and chronic kidney disease (CKD) have been sparse.
 “It is well known that certain active ingredients of the plants of Cannabis genus, i.e., the “phytocannabinoids” [pCBs; e.g., (−)-trans-Δ9-tetrahydrocannabinol (THC), (−)-cannabidiol, etc.] can influence a wide array of biological processes, and the human body is able to produce endogenous analogs of these substances [“endocannabinoids” (eCB), e.g., arachidonoylethanolamine (anandamide, AEA), 2-arachidonoylglycerol (2-AG), etc.]. These ligands, together with multiple receptors (e.g., CB1 and CB2 cannabinoid receptors, etc.), and a complex enzyme and transporter apparatus involved in the synthesis and degradation of the ligands constitute the endocannabinoid system (ECS), a recently emerging regulator of several physiological processes. The ECS is widely expressed in the human body, including several members of the innate and adaptive immune system, where eCBs, as well as several pCBs were shown to deeply influence immune functions thereby regulating inflammation, autoimmunity, antitumor, as well as antipathogen immune responses, etc. Based on this knowledge, many in vitro and in vivo studies aimed at exploiting the putative therapeutic potential of cannabinoid signaling in inflammation-accompanied diseases (e.g., multiple sclerosis) or in organ transplantation, and to dissect the complex immunological effects of medical and “recreational” marijuana consumption. Thus, the objective of the current article is (i) to summarize the most recent findings of the field; (ii) to highlight the putative therapeutic potential of targeting cannabinoid signaling; (iii) to identify open questions and key challenges; and (iv) to suggest promising future directions for cannabinoid-based drug development.
“It is well known that certain active ingredients of the plants of Cannabis genus, i.e., the “phytocannabinoids” [pCBs; e.g., (−)-trans-Δ9-tetrahydrocannabinol (THC), (−)-cannabidiol, etc.] can influence a wide array of biological processes, and the human body is able to produce endogenous analogs of these substances [“endocannabinoids” (eCB), e.g., arachidonoylethanolamine (anandamide, AEA), 2-arachidonoylglycerol (2-AG), etc.]. These ligands, together with multiple receptors (e.g., CB1 and CB2 cannabinoid receptors, etc.), and a complex enzyme and transporter apparatus involved in the synthesis and degradation of the ligands constitute the endocannabinoid system (ECS), a recently emerging regulator of several physiological processes. The ECS is widely expressed in the human body, including several members of the innate and adaptive immune system, where eCBs, as well as several pCBs were shown to deeply influence immune functions thereby regulating inflammation, autoimmunity, antitumor, as well as antipathogen immune responses, etc. Based on this knowledge, many in vitro and in vivo studies aimed at exploiting the putative therapeutic potential of cannabinoid signaling in inflammation-accompanied diseases (e.g., multiple sclerosis) or in organ transplantation, and to dissect the complex immunological effects of medical and “recreational” marijuana consumption. Thus, the objective of the current article is (i) to summarize the most recent findings of the field; (ii) to highlight the putative therapeutic potential of targeting cannabinoid signaling; (iii) to identify open questions and key challenges; and (iv) to suggest promising future directions for cannabinoid-based drug development.