 “Positive effect of some cannabinoids in the treatment and prophylaxis of a wide variety of oxidation-associated diseases and growing popularity of supplements containing cannabinoids, mainly cannabinoid oils (e.g. CBD oil, CBG oil), in the self-medication of humans cause a growing interest in the antioxidant properties of these compounds, especially those not showing psychotropic effects.
“Positive effect of some cannabinoids in the treatment and prophylaxis of a wide variety of oxidation-associated diseases and growing popularity of supplements containing cannabinoids, mainly cannabinoid oils (e.g. CBD oil, CBG oil), in the self-medication of humans cause a growing interest in the antioxidant properties of these compounds, especially those not showing psychotropic effects.
Herein, we report the antioxidant activity of cannabigerol (CBG), cannabidiol (CBD), Δ9-tetrahydrocannabinol (Δ9-THC), cannabinol (CBN), cannabigerolic acid (CBGA), cannabinolic acid (CBDA) and Δ9-tetrahydrocannabinolic acid (Δ9-THCA) estimated by spectrophotometric methods: ABTS, DPPH, ORAC, beta-carotene CUPRAC and FRAP.
The presented data prove that all the examined cannabinoids exhibit antioxidant activity manifested in their ability to scavenge free radicals, to prevent the oxidation process and to reduce metal ions. Although the intensity of these activities is not the same for the individual cannabinoids it is comparable for all of them with that of E vitamin.”
“The present paper discusses the antioxidant properties of CBG, CBN, CBDA, CBGA and Δ9-THCA which, beside CBD and Δ9-THC, are also supposed to be bioactive compounds useful in the therapeutic treatment of different diseases. According to the literature, CBD and Δ9-THC exhibit strong antioxidant activity, stronger than vitamins C, A and E.
The presented data prove that all the examined cannabinoids – CBG, CBD, Δ9-THC, CBN, CBGA CBDA and Δ9-THCA – exhibit antioxidant activity manifesting itself in their ability to scavenge free radicals, to protect oxidation process and to reduce metal ions. Although, the intensity of these activities for individual cannabinoids is not the same, it is generally comparable to that of E vitamin.” https://www.sciencedirect.com/science/article/pii/S0367326X21000903?via%3Dihub

 “Salicylic acid (SA) is a plant hormone which plays a crucial role in the plant defense against various pathogens and abiotic stresses. Increasing reports suggest that this phenolic compound and its derivatives, collectively termed salicylates, not only regulate plant defense but also have beneficial effects on human health. Both natural and synthetic salicylates are known to have multiple targets in humans, thereby exhibiting various appreciating pharmacological roles, including anti-inflammatory, anticancer, neuroprotective, antidiabetic effects, and so on. The role of some salicylates, such as acetylsalicylic acid (aspirin), 5-aminosalicylic acid (mesalazine), and amorfrutins in human diseases has been well studied in vitro. However, their clinical significance in different diseases is largely unknown. Based on recent studies, five natural salicylates, including amorfrutin, ginkgolic acid, grifolic acid, tetrahydrocannabinolic acid, and cannabidiolic acid, showed potential roles in different challenging human diseases. This review summarizes together some of the recent information on multitarget regulatory activities of these natural salicylates and their pharmacological roles in human health.”
“Salicylic acid (SA) is a plant hormone which plays a crucial role in the plant defense against various pathogens and abiotic stresses. Increasing reports suggest that this phenolic compound and its derivatives, collectively termed salicylates, not only regulate plant defense but also have beneficial effects on human health. Both natural and synthetic salicylates are known to have multiple targets in humans, thereby exhibiting various appreciating pharmacological roles, including anti-inflammatory, anticancer, neuroprotective, antidiabetic effects, and so on. The role of some salicylates, such as acetylsalicylic acid (aspirin), 5-aminosalicylic acid (mesalazine), and amorfrutins in human diseases has been well studied in vitro. However, their clinical significance in different diseases is largely unknown. Based on recent studies, five natural salicylates, including amorfrutin, ginkgolic acid, grifolic acid, tetrahydrocannabinolic acid, and cannabidiolic acid, showed potential roles in different challenging human diseases. This review summarizes together some of the recent information on multitarget regulatory activities of these natural salicylates and their pharmacological roles in human health.”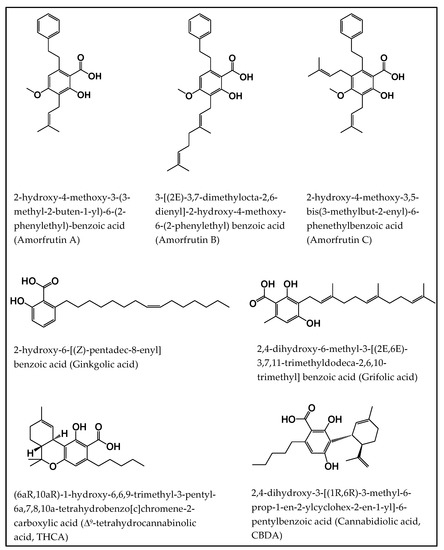


 “Δ9‐THCA‐A, the precursor of Δ9‐THC, is a non‐psychotropic phytocannabinoid that shows PPARγ agonistic activity. Herein, we investigated Δ9‐THCA ability to modulate classic cannabinoid receptors (CB1 and CB2) and evaluated its anti‐arthritis activity.
“Δ9‐THCA‐A, the precursor of Δ9‐THC, is a non‐psychotropic phytocannabinoid that shows PPARγ agonistic activity. Herein, we investigated Δ9‐THCA ability to modulate classic cannabinoid receptors (CB1 and CB2) and evaluated its anti‐arthritis activity.
 “This study evaluated the potential of combined cannabis constituents to reduce nausea.
“This study evaluated the potential of combined cannabis constituents to reduce nausea.


