 “Cannabis has long been known to limit or prevent nausea and vomiting, lack of appetite, and pain. For this reason, cannabinoids have been successfully used in the treatment of some of the unwanted side effects caused by cancer chemotherapy.
“Cannabis has long been known to limit or prevent nausea and vomiting, lack of appetite, and pain. For this reason, cannabinoids have been successfully used in the treatment of some of the unwanted side effects caused by cancer chemotherapy.
Besides their palliative effects, research from the past two decades has demonstrated their promising potential as antitumor agents in a wide variety of tumors.
Cannabinoids of endogenous, phytogenic, and synthetic nature have been shown to impact the proliferation of cancer through the modulation of different proteins involved in the endocannabinoid system such as the G protein-coupled receptors CB1, CB2, and GRP55, the ionotropic receptor TRPV1, or the fatty acid amide hydrolase (FAAH).
In this article, we aim to structurally classify the antitumor cannabinoid chemotypes described so far according to their targets and types of cancer. In a drug discovery approach, their in silico pharmacokinetic profile has been evaluated in order to identify appropriate drug-like profiles, which should be taken into account for further progress toward the clinic.
This analysis may provide structural insights into the selection of specific cannabinoid scaffolds for the development of antitumor drugs for the treatment of particular types of cancer.” https://www.ncbi.nlm.nih.gov/pubmed/31214034
“Antitumor effects of cannabidiol” http://www.ncbi.nlm.nih.gov/pubmed/14617682
“Anti-tumour actions of cannabinoids.” https://www.ncbi.nlm.nih.gov/pubmed/30019449
“Extensive preclinical research has demonstrated that cannabinoids, the active ingredients of Cannabis sativa, trigger antitumor responses in different models of cancer.” https://www.ncbi.nlm.nih.gov/pubmed/29940172




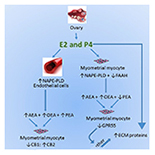
 “New neurons are continuously produced by neural stem cells (NSCs) within the adult hippocampus. Numerous diseases, including major depressive disorder and HIV-1 associated neurocognitive disorder, are associated with decreased rates of adult neurogenesis. A hallmark of these conditions is a chronic release of neuroinflammatory mediators by activated resident glia.
Recent studies have shown a neuroprotective role on NSCs of cannabinoid receptor activation. Yet, little is known about the effects of GPR55, a candidate cannabinoid receptor, activation on reductions of neurogenesis in response to inflammatory insult.
In the present study, we examined NSCs exposed to IL-1β in vitro to assess inflammation-caused effects on NSC differentiation and the ability of GPR55 agonists to attenuate NSC injury.
Taken together, these results suggest a neuroprotective role of GPR55 activation on NSCs in vitro and in vivo and that GPR55 provides a novel therapeutic target against negative regulation of hippocampal neurogenesis by inflammatory insult.”
“New neurons are continuously produced by neural stem cells (NSCs) within the adult hippocampus. Numerous diseases, including major depressive disorder and HIV-1 associated neurocognitive disorder, are associated with decreased rates of adult neurogenesis. A hallmark of these conditions is a chronic release of neuroinflammatory mediators by activated resident glia.
Recent studies have shown a neuroprotective role on NSCs of cannabinoid receptor activation. Yet, little is known about the effects of GPR55, a candidate cannabinoid receptor, activation on reductions of neurogenesis in response to inflammatory insult.
In the present study, we examined NSCs exposed to IL-1β in vitro to assess inflammation-caused effects on NSC differentiation and the ability of GPR55 agonists to attenuate NSC injury.
Taken together, these results suggest a neuroprotective role of GPR55 activation on NSCs in vitro and in vivo and that GPR55 provides a novel therapeutic target against negative regulation of hippocampal neurogenesis by inflammatory insult.”
 “The life expectancy for pancreatic cancer patients has seen no substantial changes in the last 40 years as very few and mostly just palliative treatments are available. As the five years survival rate remains around 5%, the identification of novel pharmacological targets and development of new therapeutic strategies are urgently needed.
Here we demonstrate that inhibition of the G protein-coupled receptor GPR55, using genetic and pharmacological approaches, reduces pancreatic cancer cell growth in vitro and in vivo and we propose that this may represent a novel strategy to inhibit pancreatic ductal adenocarcinoma (PDAC) progression.
Specifically, we show that genetic ablation of Gpr55 in the KRASWT/G12D/TP53WT/R172H/Pdx1-Cre+/+ (KPC) mouse model of PDAC significantly prolonged survival.
Importantly, KPC mice treated with a combination of the GPR55 antagonist Cannabidiol (CBD) and gemcitabine (GEM, one of the most used drugs to treat PDAC), survived nearly three times longer compared to mice treated with vehicle or GEM alone.
Mechanistically, knockdown or pharmacologic inhibition of GPR55 reduced anchorage-dependent and independent growth, cell cycle progression, activation of mitogen-activated protein kinase (MAPK) signalling and protein levels of ribonucleotide reductases in PDAC cells. Consistent with this, genetic ablation of Gpr55 reduced proliferation of tumour cells, MAPK signalling and ribonucleotide reductase M1 levels in KPC mice.
Combination of CBD and GEM inhibited tumour cell proliferation in KPC mice and it opposed mechanisms involved in development of resistance to GEM in vitro and in vivo. Finally, we demonstrate that the tumour suppressor p53 regulates GPR55 protein expression through modulation of the microRNA miR34b-3p.
Our results demonstrate the important role played by GPR55 downstream of p53 in PDAC progression. Moreover our data indicate that combination of CBD and GEM, both currently approved for medical use, might be tested in clinical trials as a novel promising treatment to improve PDAC patients’ outcome.”
“The life expectancy for pancreatic cancer patients has seen no substantial changes in the last 40 years as very few and mostly just palliative treatments are available. As the five years survival rate remains around 5%, the identification of novel pharmacological targets and development of new therapeutic strategies are urgently needed.
Here we demonstrate that inhibition of the G protein-coupled receptor GPR55, using genetic and pharmacological approaches, reduces pancreatic cancer cell growth in vitro and in vivo and we propose that this may represent a novel strategy to inhibit pancreatic ductal adenocarcinoma (PDAC) progression.
Specifically, we show that genetic ablation of Gpr55 in the KRASWT/G12D/TP53WT/R172H/Pdx1-Cre+/+ (KPC) mouse model of PDAC significantly prolonged survival.
Importantly, KPC mice treated with a combination of the GPR55 antagonist Cannabidiol (CBD) and gemcitabine (GEM, one of the most used drugs to treat PDAC), survived nearly three times longer compared to mice treated with vehicle or GEM alone.
Mechanistically, knockdown or pharmacologic inhibition of GPR55 reduced anchorage-dependent and independent growth, cell cycle progression, activation of mitogen-activated protein kinase (MAPK) signalling and protein levels of ribonucleotide reductases in PDAC cells. Consistent with this, genetic ablation of Gpr55 reduced proliferation of tumour cells, MAPK signalling and ribonucleotide reductase M1 levels in KPC mice.
Combination of CBD and GEM inhibited tumour cell proliferation in KPC mice and it opposed mechanisms involved in development of resistance to GEM in vitro and in vivo. Finally, we demonstrate that the tumour suppressor p53 regulates GPR55 protein expression through modulation of the microRNA miR34b-3p.
Our results demonstrate the important role played by GPR55 downstream of p53 in PDAC progression. Moreover our data indicate that combination of CBD and GEM, both currently approved for medical use, might be tested in clinical trials as a novel promising treatment to improve PDAC patients’ outcome.”