
“Glioblastoma (GBM) is the most frequent malignant tumor of the central nervous system in humans with a median survival time of less than 15 months.
∆9-Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best-characterized components of Cannabis sativa plants with modulating effects on cannabinoid receptors 1 and 2 (CB1 and CB2) and on orphan receptors such as GPR18 or GPR55. Previous studies have demonstrated anti-tumorigenic effects of THC and CBD in several tumor entities including GBM, mostly mediated via CB1 or CB2.
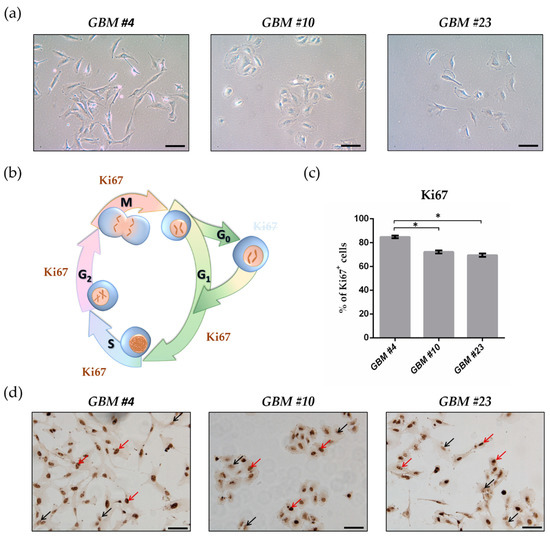
In this study, we investigated the non-CB1/CB2 effects of THC on the cell cycle of GBM cells isolated from human tumor samples.
Cell cycle entry was measured after 24 h upon exposure by immunocytochemical analysis of Ki67 as proliferation marker. The Ki67-reducing effect of THC was abolished in the presence of CBD, whereas CBD alone did not cause any changes. To identify the responsible receptor for THC effects, we first characterized the cells regarding their expression of different cannabinoid receptors: CB1, CB2, GPR18, and GPR55. Secondly, the receptors were pharmacologically blocked by application of their selective antagonists AM281, AM630, O-1918, and CID16020046 (CID), respectively. All examined cells expressed the receptors, but only in presence of the GPR55 antagonist CID was the THC effect diminished. Stimulation with the GPR55 agonist lysophosphatidylinositol (LPI) revealed similar effects as obtained for THC. The LPI effects were also inhibited by CBD and CID, confirming a participation of GPR55 and suggesting its involvement in modifying the cell cycle of patient-derived GBM cells.”
https://pubmed.ncbi.nlm.nih.gov/33802282/
https://www.mdpi.com/2072-6694/13/5/1064


 “Cannabidiol (CBD) has been shown to slow cancer cell growth and is toxic to human glioblastoma cell lines. Thus, CBD could be an effective therapeutic for glioblastoma.
“Cannabidiol (CBD) has been shown to slow cancer cell growth and is toxic to human glioblastoma cell lines. Thus, CBD could be an effective therapeutic for glioblastoma.
 “Preclinical data suggest some cannabinoids may exert antitumour effects against glioblastoma (GBM). Safety and preliminary efficacy of nabiximols oromucosal cannabinoid spray plus dose-intense temozolomide (DIT) was evaluated in patients with first recurrence of GBM.
“Preclinical data suggest some cannabinoids may exert antitumour effects against glioblastoma (GBM). Safety and preliminary efficacy of nabiximols oromucosal cannabinoid spray plus dose-intense temozolomide (DIT) was evaluated in patients with first recurrence of GBM.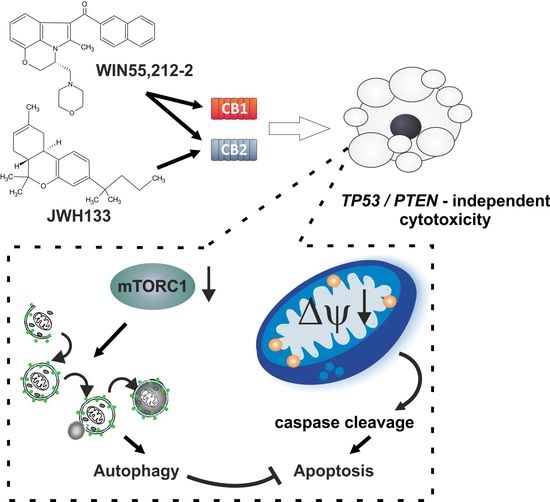
 “Glioblastoma is the most aggressive cancer among primary brain tumours. As with other cancers, the incidence of glioblastoma is increasing; despite modern therapies, the overall mean survival of patients post-diagnosis averages around 16 months, a figure that has not changed in many years. Cannabigerol (CBG) has only recently been reported to prevent the progression of certain carcinomas and has not yet been studied in glioblastoma. Here, we have compared the cytotoxic, apoptotic, and anti-invasive effects of the purified natural cannabinoid CBG together with CBD and THC on established differentiated glioblastoma tumour cells and glioblastoma stem cells. CBG and THC reduced the viability of both types of cells to a similar extent, whereas combining CBD with CBG was more efficient than with THC. CBD and CBG, both alone and in combination, induced caspase-dependent cell apoptosis, and there was no additive THC effect. Of note, CBG inhibited glioblastoma invasion in a similar manner to CBD and the chemotherapeutic temozolomide. We have demonstrated that THC has little added value in combined-cannabinoid glioblastoma treatment, suggesting that this psychotropic cannabinoid should be replaced with CBG in future clinical studies of glioblastoma therapy.”
“Glioblastoma is the most aggressive cancer among primary brain tumours. As with other cancers, the incidence of glioblastoma is increasing; despite modern therapies, the overall mean survival of patients post-diagnosis averages around 16 months, a figure that has not changed in many years. Cannabigerol (CBG) has only recently been reported to prevent the progression of certain carcinomas and has not yet been studied in glioblastoma. Here, we have compared the cytotoxic, apoptotic, and anti-invasive effects of the purified natural cannabinoid CBG together with CBD and THC on established differentiated glioblastoma tumour cells and glioblastoma stem cells. CBG and THC reduced the viability of both types of cells to a similar extent, whereas combining CBD with CBG was more efficient than with THC. CBD and CBG, both alone and in combination, induced caspase-dependent cell apoptosis, and there was no additive THC effect. Of note, CBG inhibited glioblastoma invasion in a similar manner to CBD and the chemotherapeutic temozolomide. We have demonstrated that THC has little added value in combined-cannabinoid glioblastoma treatment, suggesting that this psychotropic cannabinoid should be replaced with CBG in future clinical studies of glioblastoma therapy.” “Cannabis has long been used for healing and recreation in several regions of the world. Over 400 bioactive constituents, including more than 100 phytocannabinoids, have been isolated from this plant. The non-psychoactive cannabidiol (CBD) and the psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) are the major and widely studied constituents from this plant.
“Cannabis has long been used for healing and recreation in several regions of the world. Over 400 bioactive constituents, including more than 100 phytocannabinoids, have been isolated from this plant. The non-psychoactive cannabidiol (CBD) and the psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) are the major and widely studied constituents from this plant. “Providers need to be better equipped to discuss medical cannabis with patients even if they are not willing to prescribe it. The oncology community would be well served to ensure that providers are aware of existing cannabis research and are able to incorporate it into their communications with patients instead of leaving patients to figure out medical cannabis on their own.”
“Providers need to be better equipped to discuss medical cannabis with patients even if they are not willing to prescribe it. The oncology community would be well served to ensure that providers are aware of existing cannabis research and are able to incorporate it into their communications with patients instead of leaving patients to figure out medical cannabis on their own.”