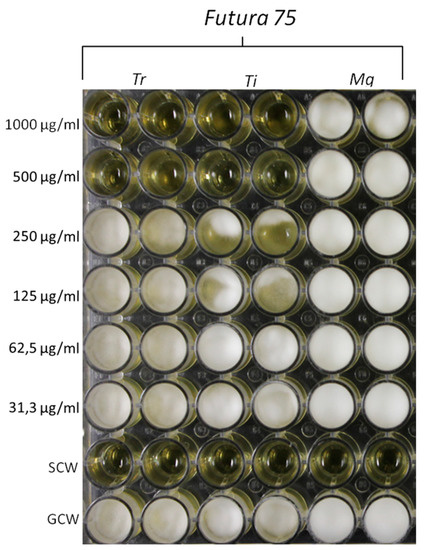
“Industrial hemp (Cannabis sativa) is traditionally cultivated as a valuable source of fibers and nutrients. Multiple studies also demonstrated antimicrobial, anti-proliferative, phytotoxic and insecticide effects of the essential oil from hemp female inflorescences. On the other side, only a few studies explored the potential pharmacological application of polar extracts from inflorescences. In the present study, we investigated the water extract from inflorescences of industrial hemp Futura 75 variety, from phytochemical and pharmacological point of view. The water extract was assayed for phenolic compound content, radical scavenger/reducing, chelating and anti-tyrosinase effects. Through an ex vivo model of toxicity induced by lipopolysaccharide (LPS) on isolated rat colon and liver, we explored the extract effects on serotonin, dopamine and kynurenine pathways and the production of prostaglandin (PG)E2. Anti-proliferative effects were also evaluated against human colon cancer HCT116 cell line. Additionally, antimycotic effects were investigated against Trichophyton rubrum, Trichophyton interdigitale, Microsporum gypseum. Finally, in silico studies, including bioinformatics, network pharmacology and docking approaches were conducted in order to predict the putative targets underlying the observed pharmacological and microbiological effects. Futura 75 water extract was able to blunt LPS-induced reduction of serotonin and increase of dopamine and kynurenine turnover, in rat colon. Additionally, the reduction of PGE2 levels was observed in both colon and liver specimens, as well. The extract inhibited the HCT116 cell viability, the growth of T. rubrum and T. interdigitale and the activity of tyrosinase, in vitro, whereas in silico studies highlighting the inhibitions of cyclooxygenase-1 (induced by carvacrol), carbonic anhydrase IX (induced by chlorogenic acid and gallic acid) and lanosterol 14-α-demethylase (induced by rutin) further support the observed pharmacological and antimycotic effects. The present findings suggest female inflorescences from industrial hemp as high quality by-products, thus representing promising sources of nutraceuticals and cosmeceuticals against inflammatory and infectious diseases.”
https://pubmed.ncbi.nlm.nih.gov/32429587/
“Concluding, the present findings highlight the potential of water extracts from industrial hemp inflorescences as sources of natural compounds that, besides the intrinsic antiradical activity, possess discrete mechanisms related to anti-inflammatory, anti-proliferative and antimycotic effects. In this context, and also in view of a more sustainable circular economy, it is desirable an improvement of industrial hemp chain production, taking into consideration the female inflorescences as high quality by-products with putative nutraceutical and cosmeceutical applications.”

 “Low tetrahydrocannabinol Cannabis sativa products, also known as hemp products, have become widely available and their use in veterinary patients has become increasingly popular. Despite prevalence of use, the veterinary literature is lacking and evidence-based resource for cannabinoid efficacy.
“Low tetrahydrocannabinol Cannabis sativa products, also known as hemp products, have become widely available and their use in veterinary patients has become increasingly popular. Despite prevalence of use, the veterinary literature is lacking and evidence-based resource for cannabinoid efficacy. “Cannabinoids are a group of structurally heterogeneous but pharmacologically related compounds, including plant-derived cannabinoids, synthetic substances and endogenous cannabinoids, such as anandamide and 2-arachidonoylglycerol.
“Cannabinoids are a group of structurally heterogeneous but pharmacologically related compounds, including plant-derived cannabinoids, synthetic substances and endogenous cannabinoids, such as anandamide and 2-arachidonoylglycerol. “In the last decade, we have observed an increased public and scientific interest in the clinical applications of medical cannabis.
“In the last decade, we have observed an increased public and scientific interest in the clinical applications of medical cannabis. “Cannabis sativa L. is a plant long used for its textile fibers, seed oil, and oleoresin with medicinal and psychoactive properties. It is the main source of phytocannabinoids, with over 100 compounds detected so far. In recent years, a lot of attention has been given to the main phytochemicals present in Cannabis sativa L., namely,
“Cannabis sativa L. is a plant long used for its textile fibers, seed oil, and oleoresin with medicinal and psychoactive properties. It is the main source of phytocannabinoids, with over 100 compounds detected so far. In recent years, a lot of attention has been given to the main phytochemicals present in Cannabis sativa L., namely, 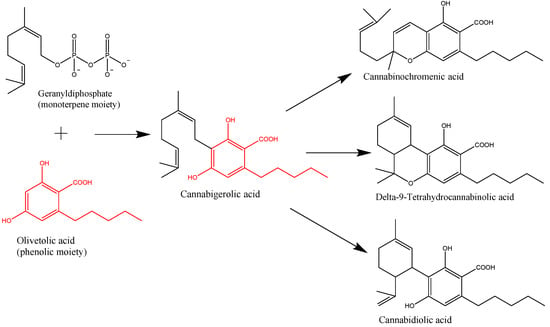
 “Several studies demonstrated that
“Several studies demonstrated that 
1098-2744/asset/olalertbanner.jpg?l=SkaBT8QEx2rBLBmQ2URf7fAaMkdr93XX6rZGHTxNciKmUq8irCOtJ8iDpNMUJn%2FA)
