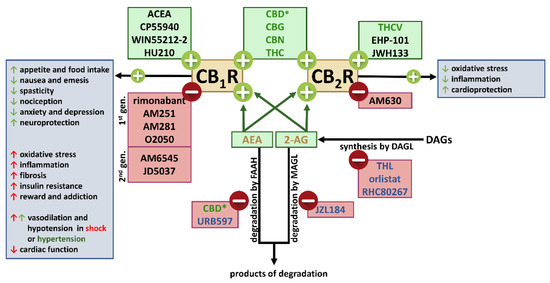
“This review is dedicated to the cross-talk between the (endo)cannabinoid and renin angiotensin systems (RAS). Activation of AT1 receptors (AT1Rs) by angiotensin II (Ang II) can release endocannabinoids that, by acting at cannabinoid CB1 receptors (CB1Rs), modify the response to AT1R stimulation.
CB1R blockade may enhance AT1R-mediated responses (mainly vasoconstrictor effects) or reduce them (mainly central nervous system-mediated effects). The final effects depend on whether stimulation of CB1Rs and AT1Rs induces opposite or the same effects. Second, CB1R blockade may diminish AT1R levels. Third, phytocannabinoids modulate angiotensin-converting enzyme-2. Additional studies are required to clarify (1) the existence of a cross-talk between the protective axis of the RAS (Ang II-AT2 receptor system or angiotensin 1-7-Mas receptor system) with components of the endocannabinoid system, (2) the influence of Ang II on constituents of the endocannabinoid system and (3) the (patho)physiological significance of AT1R-CB1R heteromerization.
As a therapeutic consequence, CB1R antagonists may influence effects elicited by the activation or blockade of the RAS; phytocannabinoids may be useful as adjuvant therapy against COVID-19; single drugs acting on the (endo)cannabinoid system (cannabidiol) and the RAS (telmisartan) may show pharmacokinetic interactions since they are substrates of the same metabolizing enzyme of the transport mechanism.”
https://pubmed.ncbi.nlm.nih.gov/35683028/
https://www.mdpi.com/1422-0067/23/11/6350