
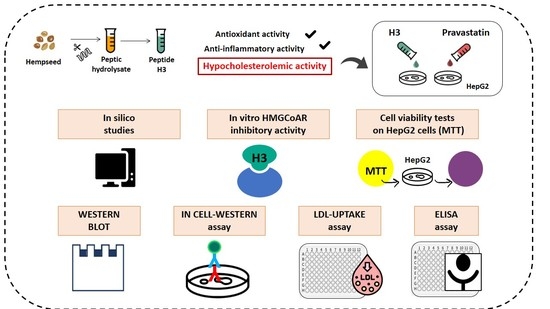
“Hempseed (Cannabis sativa) protein is an important source of bioactive peptides. H3 (IGFLIIWV), a transepithelial transported intestinal peptide obtained from the hydrolysis of hempseed protein with pepsin, carries out antioxidant and anti-inflammatory activities in HepG2 cells. In this study, the main aim was to assess its hypocholesterolemic effects at a cellular level and the mechanisms behind this health-promoting activity. The results showed that peptide H3 inhibited the 3-hydroxy-3-methylglutaryl co-enzyme A reductase (HMGCoAR) activity in vitro in a dose-dependent manner with an IC50 value of 59 μM. Furthermore, the activation of the sterol regulatory element binding proteins (SREBP)-2 transcription factor, followed by the increase of low-density lipoprotein (LDL) receptor (LDLR) protein levels, was observed in human hepatic HepG2 cells treated with peptide H3 at 25 µM. Meanwhile, peptide H3 regulated the intracellular HMGCoAR activity through the increase of its phosphorylation by the activation of AMP-activated protein kinase (AMPK)-pathways. Consequently, the augmentation of the LDLR localized on the cellular membranes led to the improved ability of HepG2 cells to uptake extracellular LDL with a positive effect on cholesterol levels. Unlike the complete hempseed hydrolysate (HP), peptide H3 can reduce the proprotein convertase subtilisin/kexin 9 (PCSK9) protein levels and its secretion in the extracellular environment via the decrease of hepatic nuclear factor 1-α (HNF1-α). Considering all these evidences, H3 may represent a new bioactive peptide to be used for the development of dietary supplements and/or peptidomimetics for cardiovascular disease (CVD) prevention.”
https://pubmed.ncbi.nlm.nih.gov/35565772/
https://www.mdpi.com/2072-6643/14/9/1804


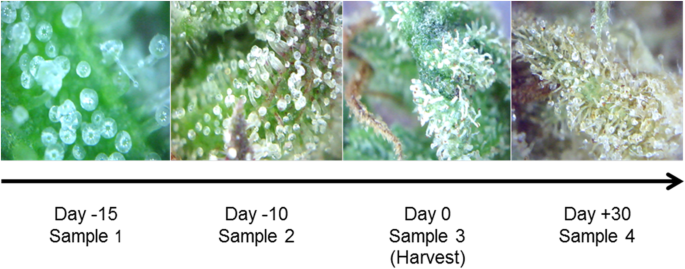

 “The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems.
“The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems.