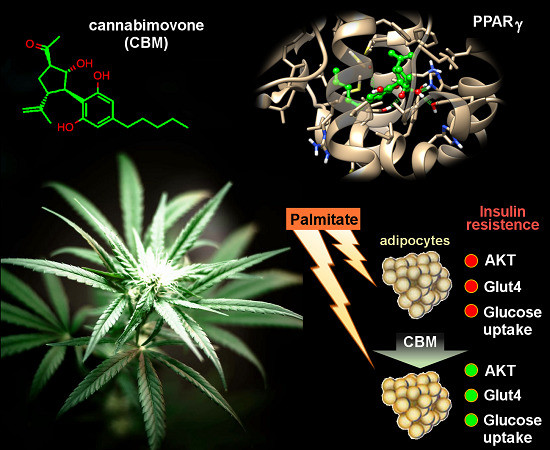
 “Phytocannabinoids (pCBs) are a large family of meroterpenoids isolated from the plant Cannabis sativa. Δ9-Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best investigated phytocannabinoids due to their relative abundance and interesting bioactivity profiles. In addition to various targets, THC and CBD are also well-known agonists of peroxisome proliferator-activated receptor gamma (PPARγ), a nuclear receptor involved in energy homeostasis and lipid metabolism. In the search of new pCBs potentially acting as PPARγ agonists, we identified cannabimovone (CBM), a structurally unique abeo-menthane pCB, as a novel PPARγ modulator via a combined computational and experimental approach. The ability of CBM to act as dual PPARγ/α agonist was also evaluated. Computational studies suggested a different binding mode toward the two isoforms, with the compound able to recapitulate the pattern of H-bonds of a canonical agonist only in the case of PPARγ. Luciferase assays confirmed the computational results, showing a selective activation of PPARγ by CBM in the low micromolar range. CBM promoted the expression of PPARγ target genes regulating the adipocyte differentiation and prevented palmitate-induced insulin signaling impairment. Altogether, these results candidate CBM as a novel bioactive compound potentially useful for the treatment of insulin resistance-related disorders.”
“Phytocannabinoids (pCBs) are a large family of meroterpenoids isolated from the plant Cannabis sativa. Δ9-Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best investigated phytocannabinoids due to their relative abundance and interesting bioactivity profiles. In addition to various targets, THC and CBD are also well-known agonists of peroxisome proliferator-activated receptor gamma (PPARγ), a nuclear receptor involved in energy homeostasis and lipid metabolism. In the search of new pCBs potentially acting as PPARγ agonists, we identified cannabimovone (CBM), a structurally unique abeo-menthane pCB, as a novel PPARγ modulator via a combined computational and experimental approach. The ability of CBM to act as dual PPARγ/α agonist was also evaluated. Computational studies suggested a different binding mode toward the two isoforms, with the compound able to recapitulate the pattern of H-bonds of a canonical agonist only in the case of PPARγ. Luciferase assays confirmed the computational results, showing a selective activation of PPARγ by CBM in the low micromolar range. CBM promoted the expression of PPARγ target genes regulating the adipocyte differentiation and prevented palmitate-induced insulin signaling impairment. Altogether, these results candidate CBM as a novel bioactive compound potentially useful for the treatment of insulin resistance-related disorders.”
https://www.ncbi.nlm.nih.gov/pubmed/32138197
https://www.mdpi.com/1420-3049/25/5/1119

1099-0690/asset/olbannerleft.jpg?v=1&s=94dd839c0b65e73a7f81fddc29bbb23abcaba5c9)