 “The level of the major endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are altered in several types of carcinomas, and are known to regulate tumor growth. Thusly, this study hypothesized that the HEp-2 human laryngeal squamous cell carcinoma (LSCC) cell line releases AEA and 2-AG, and aimed to determine if their exogenous supplementation has an anti-proliferative effect in vitro.
“The level of the major endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are altered in several types of carcinomas, and are known to regulate tumor growth. Thusly, this study hypothesized that the HEp-2 human laryngeal squamous cell carcinoma (LSCC) cell line releases AEA and 2-AG, and aimed to determine if their exogenous supplementation has an anti-proliferative effect in vitro.
In this in vitro observational study a commercial human LSCC cell line (HEp-2) was used to test for endogenous AEA and 2-AG release via liquid chromatography-tandem mass spectrometry (LC-MS/MS). The anti-proliferative effect of AEA and 2-AG supplementation was evaluated via WST-1 proliferation assay. It was observed that the HEp-2 LSCC cell line released AEA and 2-AG; the median quantity of AEA released was 15.69 ng mL-1 (range: 14.55-15.95 ng mL-1) and the median quantity of 2-AG released was 2.72 ng -1 (range: 2.67-2.74 ng mL-1). Additionally, both AEA and 2-AG exhibited an anti-proliferative effect. The anti-proliferative effect of 2-AG was stronger than that of AEA. These findings suggest that AEA might function via a CB1 receptor-independent pathway and that 2-AG might function via a CB2-dependent pathway.
The present findings show that the HEp-2 LSCC cell line releases the major endocannabinoids AEA and 2-AG, and that their supplementation inhibits tumor cell proliferation in vitro. Thus, cannabinoid ligands might represent novel drug candidates for laryngeal cancers, although functional in vivo studies are required in order to validate their potency.”
https://pubmed.ncbi.nlm.nih.gov/33797678/
https://link.springer.com/article/10.1007/s10561-021-09917-9

 “Gynaecological cancers can be primary neoplasms, originating either from the reproductive tract or the products of conception, or secondary neoplasms, representative of metastatic disease. For some of these cancers, the exact causes are unknown; however, it is recognised that the precise aetiopathogeneses for most are multifactorial and include exogenous (such as diet) and endogenous factors (such as genetic predisposition), which mutually interact in a complex manner.
“Gynaecological cancers can be primary neoplasms, originating either from the reproductive tract or the products of conception, or secondary neoplasms, representative of metastatic disease. For some of these cancers, the exact causes are unknown; however, it is recognised that the precise aetiopathogeneses for most are multifactorial and include exogenous (such as diet) and endogenous factors (such as genetic predisposition), which mutually interact in a complex manner.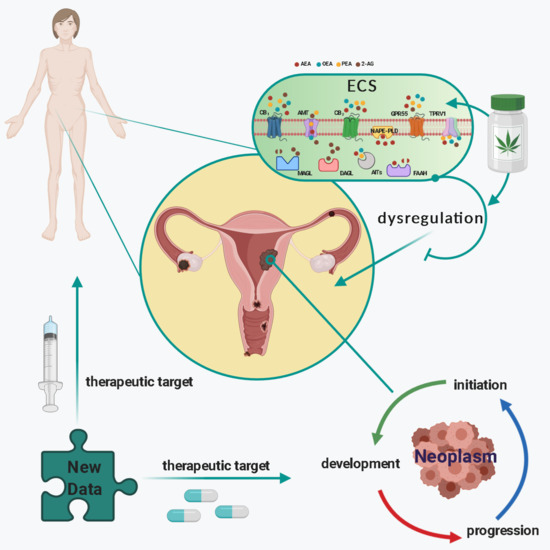
 “Leukocytes are part of the tumor microenvironment (TME) and are critical determinants of tumor progression. Because of the immunoregulatory properties of cannabinoids, the endocannabinoid system (ECS) may have an important role in shaping the TME.
“Leukocytes are part of the tumor microenvironment (TME) and are critical determinants of tumor progression. Because of the immunoregulatory properties of cannabinoids, the endocannabinoid system (ECS) may have an important role in shaping the TME.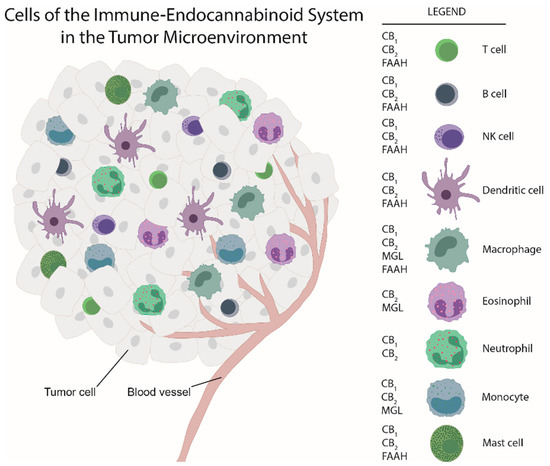
 “Coronavirus disease 2019 (COVID-19) is a highly infectious respiratory disease caused by the severe acute respiratory syndrome coronavirus 2. A significant proportion of COVID-19 patients develop Acute Respiratory Distress Syndrome (ARDS) resulting from hyperactivation of the immune system and cytokine storm, which leads to respiratory and multi-organ failure, and death. Currently, there are no effective treatments against hyperimmune syndrome and ARDS.
“Coronavirus disease 2019 (COVID-19) is a highly infectious respiratory disease caused by the severe acute respiratory syndrome coronavirus 2. A significant proportion of COVID-19 patients develop Acute Respiratory Distress Syndrome (ARDS) resulting from hyperactivation of the immune system and cytokine storm, which leads to respiratory and multi-organ failure, and death. Currently, there are no effective treatments against hyperimmune syndrome and ARDS. “The endocannabinoid (eCB) system encompasses the eCBs anandamide and 2-arachidonoylglycerol, their anabolic/catabolic enzymes, and the cannabinoid CB1 and CB2 receptors. Its expansion to include several eCB-like lipid mediators, their metabolic enzymes, and their molecular targets, forms the endocannabinoidome (eCBome).
“The endocannabinoid (eCB) system encompasses the eCBs anandamide and 2-arachidonoylglycerol, their anabolic/catabolic enzymes, and the cannabinoid CB1 and CB2 receptors. Its expansion to include several eCB-like lipid mediators, their metabolic enzymes, and their molecular targets, forms the endocannabinoidome (eCBome).
