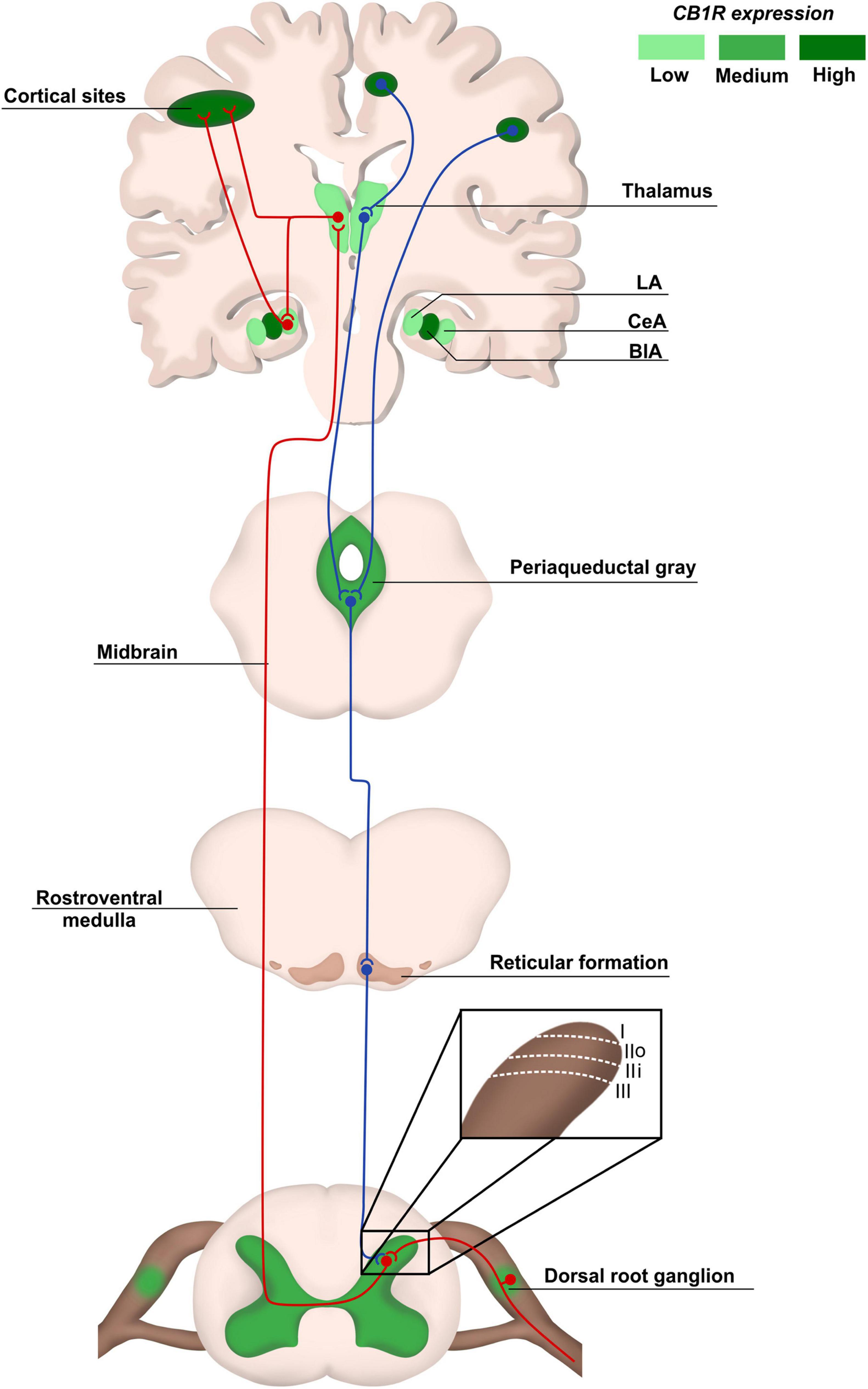
“While effective in treating abdominal pain, opioids have significant side effects. Recent legalization of cannabis will likely promote use of cannabinoids as an adjunct or alternative to opioids, despite a lack of evidence.
We aimed to investigate if cannabinoids inhibit mouse colonic nociception, alone or in combination with opioids at low doses.
Experiments were performed on C57BL/6 male and female mice. Visceral nociception was evaluated by measuring visceromotor responses (VMR), afferent nerve mechanosensitivity in flat-sheet colon preparations, and excitability of isolated dorsal root ganglion (DRG) neurons. Blood oxygen saturation, locomotion and defecation were measured to evaluate side effects.
An agonist of cannabinoid 1 receptor (CB1R), arachidonyl-2′-chloroethylamide (ACEA), dose-dependently decreased VMR. ACEA and HU-210 (another CB1R agonist) also attenuated colonic afferent nerve mechanosensitivity. Additionally, HU-210 concentration-dependently decreased DRG neuron excitability, which was reversed by the CB1R antagonist AM-251. Conversely, cannabinoid 2 receptor (CB2R) agonists did not attenuate VMR, afferent nerve mechanosensitivity or DRG neuron excitability.
Combination of sub-analgesic doses of CB1R and µ-opioid receptor (MOR) agonists decreased VMR; importantly, this analgesic effect was preserved after 6 days of twice daily treatment. This combination also attenuated afferent nerve mechanosensitivity and DRG neuron excitability, which was inhibited by neuronal nitric oxide synthase (nNOS) and guanylate cyclase inhibitors. This combination avoided side effects (decreased oxygen saturation and colonic transit) caused by analgesic dose of morphine. Activation of CB1R, but not CB2R, decreased colonic nociception both alone and in synergy with MOR.
Thus, CB1R agonists may enable opioid dose reduction and avoid opioid-related side effects.
SIGNIFICANCE STATEMENTOne of the most cited needs for patients with abdominal pain are safe and effective treatment options. The effectiveness of opioids in the management of abdominal pain is undermined by severe adverse side effects. Therefore, strategies to replace opioids or reduce the doses of opioids to suppress abdominal pain is needed. This study in mice demonstrates that cannabinoid 1 receptor (CB1R) agonists inhibit visceral sensation. Furthermore, a combination of sub-analgesic doses of µ-opioid receptor agonist and CB1R agonist markedly reduce abdominal pain without causing the side effects of high dose opioids. Thus, CB1R agonists, alone or in combination with low-dose opioids, may be a novel and safe treatment strategy for abdominal pain.”
https://pubmed.ncbi.nlm.nih.gov/35790401/