
“Intestinal inflammation is mediated by a subset of cells populating the intestine, such as enteric glial cells (EGC) and macrophages. Different studies indicate that phytocannabinoids could play a possible role in the treatment of inflammatory bowel disease (IBD) by relieving the symptoms involved in the disease.
Phytocannabinoids act through the endocannabinoid system, which is distributed throughout the mammalian body in the cells of the immune system and in the intestinal cells. Our in vitro study analyzed the putative anti-inflammatory effect of nine selected pure cannabinoids in J774A1 macrophage cells and EGCs triggered to undergo inflammation with lipopolysaccharide (LPS). The anti-inflammatory effect of several phytocannabinoids was measured by their ability to reduce TNFα transcription and translation in J774A1 macrophages and to diminish S100B and GFAP secretion and transcription in EGCs.
Our results demonstrate that THC at the lower concentrations tested exerted the most effective anti-inflammatory effect in both J774A1 macrophages and EGCs compared to the other phytocannabinoids tested herein.
We then performed RNA-seq analysis of EGCs exposed to LPS in the presence or absence of THC or THC-COOH. Transcriptomic analysis of these EGCs revealed 23 differentially expressed genes (DEG) compared to the treatment with only LPS. Pretreatment with THC resulted in 26 DEG, and pretreatment with THC-COOH resulted in 25 DEG. To evaluate which biological pathways were affected by the different phytocannabinoid treatments, we used the Ingenuity platform. We show that THC treatment affects the mTOR and RAR signaling pathway, while THC-COOH mainly affects the IL6 signaling pathway.”






 “The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems.
“The Endocannabinoid System (ECS) is primarily responsible for maintaining homeostasis, a balance in internal environment (temperature, mood, and immune system) and energy input and output in living, biological systems. 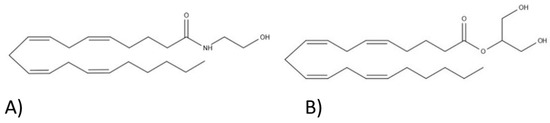
 “The relevance and incidence of intestinal bowel diseases (IBD) have been increasing over the last 50 years and the current therapies are characterized by severe side effects, making essential the development of new strategies that combine efficacy and safety in the management of human IBD. Herbal products are highly considered in research aimed at discovering new approaches for IBD therapy and, among others,
“The relevance and incidence of intestinal bowel diseases (IBD) have been increasing over the last 50 years and the current therapies are characterized by severe side effects, making essential the development of new strategies that combine efficacy and safety in the management of human IBD. Herbal products are highly considered in research aimed at discovering new approaches for IBD therapy and, among others, 
