“The structures of 2 new polyhydroxylated cannabinoids, (+/-)9,10-dihydroxy-delta6a(10a)-tetrahydrocannabinol and (+/-)8,9-dihydroxy-delta6a(10a)-tetrahydrocannabinol, obtained from a hexane extract of an Indian Cannabis variant were determined by spectral means and correlation with cannabinol.”
Monthly Archives: May 2015
Cannabinoid compounds in South African Cannabis sativa L.
“Dagga (Cannabis sativa L.) samples were collected from various geographical regions of South Africa. These were classified into age, sex and plant part and the cannabinoids were analysed quantitatively by gas-liquid chromatography and mass spectrometry. Analytical results show that there appears to be at least three chemovariants of Cannabis sativa growing in South Africa with respect to relative cannabinoid content. One of these variants appears to be unique to Southern Africa. It also appears that South African C. sativa ranks among the world’s more potent C. sativa variants in terms of its delta 9-tetrahydrocannabinol content.”
Cannabiripsol: a novel Cannabis constituent.
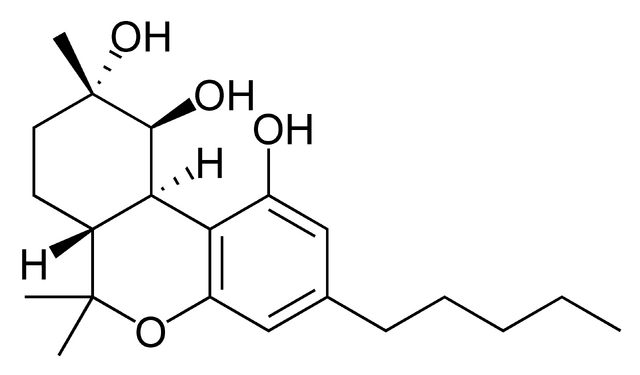
“Cannabiripsol [(-) (6aR, 9S, 10S, 10aR)9,10-dihydroxy-hexahydrocannabinol] (1), a new cannabinoid was isolated from a South African Cannabis variant. The structure was determined by spectral means and by synthesis.”
Production of Δ9-tetrahydrocannabinolic acid from cannabigerolic acid by whole cells of Pichia (Komagataella) pastoris expressing Δ9-tetrahydrocannabinolic acid synthase from Cannabis sativa L.
“The Δ9-tetrahydrocannabinolic acid synthase (THCAS) from Cannabis sativa was expressed intracellularly in different organisms to investigate the potential of a biotechnological production of Δ9-tetrahydrocannabinolic acid (THCA) using whole cells…
CONCLUSION:
Whole cells of P. pastoris offer the capability of synthesizing pharmaceutical THCA production.”
Isolation and Pharmacological Evaluation of Minor Cannabinoids from High-Potency Cannabis sativa.
“Seven new naturally occurring hydroxylated cannabinoids (1-7), along with the known cannabiripsol (8), have been isolated from the aerial parts of high-potency Cannabis sativa.
The structures of the new compounds were determined by 1D and 2D NMR spectroscopic analysis, GC-MS, and HRESIMS as 8α-hydroxy-Δ9-tetrahydrocannabinol (1), 8β-hydroxy-Δ9-tetrahydrocannabinol (2), 10α-hydroxy-Δ8-tetrahydrocannabinol (3), 10β-hydroxy-Δ8-tetrahydrocannabinol (4), 10α-hydroxy-Δ9,11-hexahydrocannabinol (5), 9β,10β-epoxyhexahydrocannabinol (6), and 11-acetoxy-Δ9-tetrahydrocannabinolic acid A (7).
The binding affinity of isolated compounds 1-8, Δ9-tetrahydrocannabinol, and Δ8-tetrahydrocannabinol toward CB1 and CB2 receptors as well as their behavioral effects in a mouse tetrad assay were studied.
The results indicated that compound 3, with the highest affinity to the CB1 receptors, exerted the most potent cannabimimetic-like actions in the tetrad assay, while compound 4 showed partial cannabimimetic actions. Compound 2, on the other hand, displayed a dose-dependent hypolocomotive effect only.”
Enhancement of endocannabinoid signaling protects against cocaine-induced neurotoxicity.
“Cocaine is an addictive substance with a potential to cause deleterious effects in the brain. The strategies for treating its neurotoxicity, however, are limited.
Evidence suggests that the endocannabinoid system exerts neuroprotective functions against various stimuli. Thus, we hypothesized that inhibition of fatty acid amide hydrolase (FAAH), the main enzyme responsible for terminating the actions of the endocannabinoid anandamide, reduces seizures and cell death in the hippocampus in a model of cocaine intoxication…
In conclusion, the pharmacological facilitation of the anandamide/CB1/PI3K signaling protects the brain against cocaine intoxication in experimental models. This strategy may be further explored in the development of treatments for drug-induced neurotoxicity.”
Cannabidiol Rescues Acute Hepatic Toxicity and Seizure Induced by Cocaine.
“Cocaine is a commonly abused illicit drug that causes significant morbidity and mortality. The most severe and common complications are seizures, ischemic strokes, myocardial infarction, and acute liver injury. Here, we demonstrated that acute cocaine intoxication promoted seizure along with acute liver damage in mice, with intense inflammatory infiltrate.
Considering the protective role of the endocannabinoid system against cell toxicity, we hypothesized that treatment with an anandamide hydrolysis inhibitor, URB597, or with a phytocannabinoid, cannabidiol (CBD), protects against cocaine toxicity.
URB597 (1.0 mg/kg) abolished cocaine-induced seizure, yet it did not protect against acute liver injury.
Using confocal liver intravital microscopy, we observed that CBD reduced acute liver inflammation and damage induced by cocaine and prevented associated seizure.
Additionally, we showed that previous liver damage induced by another hepatotoxic drug (acetaminophen) increased seizure and lethality induced by cocaine intoxication, linking hepatotoxicity to seizure dynamics.
These findings suggest that activation of cannabinoid system may have protective actions on both liver and brain induced by cocaine, minimizing inflammatory injury promoted by cocaine, supporting its further clinical application in the treatment of cocaine abuse.”
Full FAAH inhibition combined with partial monoacylglycerol lipase inhibition: Augmented and sustained antinociceptive effects with negligible cannabimimetic side effects in mice.
“Inhibition of fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MAGL), the primary hydrolytic enzymes for the respective endocannabinoids, N-arachidonoylethanolamine (AEA) and 2-arachidonylglycerol (2-AG), produces antinociception, but with minimal cannabimimetic side effects.
Although selective inhibitors of either enzyme often show partial efficacy in various nociceptive models, their combined blockade elicits augmented antinociceptive effects, but side effects emerge. Moreover, complete and prolonged MAGL blockade leads to CB1 receptor functional tolerance, which represents another challenge in this potential therapeutic strategy.
Therefore, the present study tested whether full FAAH inhibition, combined with partial MAGL inhibition, would produce sustained antinociceptive effects with minimal cannabimimetic side effects…
Thus, full FAAH inhibition combined with partial MAGL inhibition reduces neuropathic and inflammatory pain states, with minimal cannabimimetic effects.”
Lipid nanoparticles as an emerging platform for cannabinoid delivery: physicochemical optimization and biocompatibility.
“This work aims at developing and optimizing a valuable oral delivery carrier for the cannabinoid derivative CB13, which presents a high therapeutic potential in chronic pain states that respond poorly to conventional analgesics, but also shows highly unfavorable physicochemical properties.
CB13-loaded lipid nanoparticles (LNP) formulations were developed…
The LNP formulation proposed proved to be a promising carrier for the oral delivery of CB13, a cannabinoid with high therapeutic potential in chronic pain states that currently lack a valid oral treatment.”
Synthetic and endogenous cannabinoids protect retinal neurons from AMPA excitotoxicity in vivo, via activation of CB1 receptors: Involvement of PI3K/Akt and MEK/ERK signaling pathways.
“Cannabinoids have been suggested to protect retinal ganglion cells in different models of toxicity…
These results suggest that endogenous and synthetic cannabinoids protect retinal amacrine neurons from AMPA excitotoxicity in vivo via a mechanism involving the CB1 receptors, and the PI3K/Akt and/or MEK/ERK1/2 signaling pathways.”
