 “Prostate cancer is the second most frequently occurring cancer diagnosed among males. Recent preclinical evidence implicates cannabinoids as powerful regulators of cell growth and differentiation. In this review, we focused on studies that demonstrated anticancer effects of cannabinoids and their possible mechanisms of action in prostate cancer. Besides the palliative effects of cannabinoids, research from the past two decades has demonstrated their promising potential as antitumor agents in a wide variety of cancers. This analysis may provide pharmacological insights into the selection of specific cannabinoids for the development of antitumor drugs for the treatment of prostate cancer.”
“Prostate cancer is the second most frequently occurring cancer diagnosed among males. Recent preclinical evidence implicates cannabinoids as powerful regulators of cell growth and differentiation. In this review, we focused on studies that demonstrated anticancer effects of cannabinoids and their possible mechanisms of action in prostate cancer. Besides the palliative effects of cannabinoids, research from the past two decades has demonstrated their promising potential as antitumor agents in a wide variety of cancers. This analysis may provide pharmacological insights into the selection of specific cannabinoids for the development of antitumor drugs for the treatment of prostate cancer.”
“Prostate cancer, after lung cancer, is the leading cause of death among men. Although the pathophysiological mechanisms and the etiological factors of prostate cancer development are still poorly understood, there are several factors associated with the risk of developing the disease such as age, family history, lifestyle-related factors (e.g., smoking, diet), and testosterone levels. Cannabinoids are an emerging class of pharmacological molecules that may exert their therapeutic effect against different cancers, including those from the prostate. Several studies have shown that various agonists are able to target cannabinoid receptors exhibited on prostate cancer cells.”
https://www.mdpi.com/2072-6694/13/16/4107


 “The liver is a key metabolic organ that is particularly sensitive to environmental factors, including UV radiation. As UV radiation induces oxidative stress and inflammation, natural compounds are under investigation as one method to counteract these consequences.
“The liver is a key metabolic organ that is particularly sensitive to environmental factors, including UV radiation. As UV radiation induces oxidative stress and inflammation, natural compounds are under investigation as one method to counteract these consequences.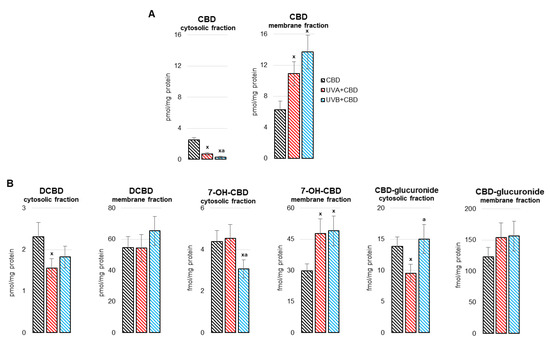
 “Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the loss of dopaminergic neurons in the Substantia Nigra pars compacta, leading to classical PD motor symptoms. Current therapies are purely symptomatic and do not modify disease progression.
“Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the loss of dopaminergic neurons in the Substantia Nigra pars compacta, leading to classical PD motor symptoms. Current therapies are purely symptomatic and do not modify disease progression.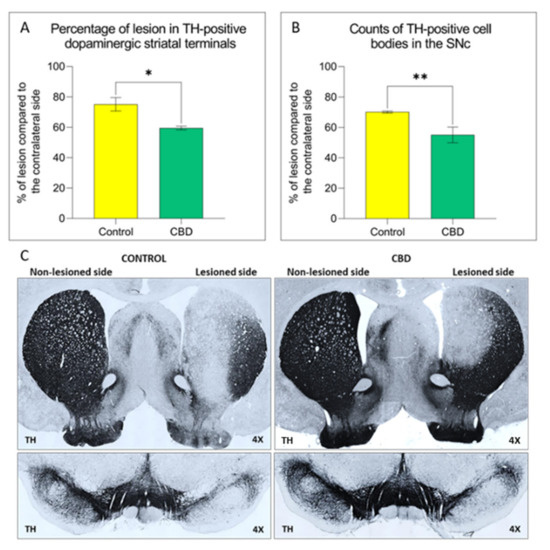
 “Obesity-related insulin resistance (IR) and attenuated brain insulin signaling are significant risk factors for neurodegenerative disorders, e.g., Alzheimer’s disease. IR and type 2 diabetes correlate with an increased concentration of sphingolipids, a class of lipids that play an essential structural role in cellular membranes and cell signaling pathways.
“Obesity-related insulin resistance (IR) and attenuated brain insulin signaling are significant risk factors for neurodegenerative disorders, e.g., Alzheimer’s disease. IR and type 2 diabetes correlate with an increased concentration of sphingolipids, a class of lipids that play an essential structural role in cellular membranes and cell signaling pathways. “Objectives: To evaluate the impact of cannabinoids on neurobehavioral outcomes in preclinical models of nontraumatic and traumatic spinal cord injury (SCI), with the aim of determining suitability for clinical trials involving SCI patients.
“Objectives: To evaluate the impact of cannabinoids on neurobehavioral outcomes in preclinical models of nontraumatic and traumatic spinal cord injury (SCI), with the aim of determining suitability for clinical trials involving SCI patients. “Background:
“Background:  “Cannabidiol is increasingly considered for treatment of a wide range of medical conditions.
“Cannabidiol is increasingly considered for treatment of a wide range of medical conditions. 
 “Despite the high incidence of traumatic brain injury (TBI), there is no universal treatment to safely treat patients. Blunt brain injuries destroy primary neural tissue that results in impaired perfusion, excessive release of glutamate, inflammation, excitotoxicity, and progressive secondary neuronal cell death.
“Despite the high incidence of traumatic brain injury (TBI), there is no universal treatment to safely treat patients. Blunt brain injuries destroy primary neural tissue that results in impaired perfusion, excessive release of glutamate, inflammation, excitotoxicity, and progressive secondary neuronal cell death.