 “Acute Respiratory Distress Syndrome (ARDS) is a life-threatening complication that can ensue following Staphylococcus aureus infection. The enterotoxin produced by these bacteria (SEB) acts as a superantigen thereby activating a large proportion of T cells leading to cytokine storm and severe lung injury.
“Acute Respiratory Distress Syndrome (ARDS) is a life-threatening complication that can ensue following Staphylococcus aureus infection. The enterotoxin produced by these bacteria (SEB) acts as a superantigen thereby activating a large proportion of T cells leading to cytokine storm and severe lung injury.
Δ9Tetrahydrocannabinol (THC), a psychoactive ingredient found in Cannabis sativa, has been shown to act as a potent anti-inflammatory agent. In the current study, we investigated the effect of THC treatment on SEB-induced ARDS in mice.
While exposure to SEB resulted in acute mortality, treatment with THC led to 100% survival of mice. THC treatment significantly suppressed the inflammatory cytokines, IFN-γ and TNF-α. Additionally, THC elevated the induction of regulatory T cells (Tregs) and their associated cytokines, IL-10 and TGF-β. Moreover, THC caused induction of Myeloid-Derived Suppressor Cells (MDSCs).
THC acted through CB2 receptor as pharmacological inhibitor of CB2 receptors blocked the anti-inflammatory effects. THC-treated mice showed significant alterations in the expression of miRNA (miRs) in the lung-infiltrated mononuclear cells (MNCs). Specifically, THC caused downregulation of let7a-5p which targeted SOCS1 and downregulation of miR-34-5p which caused increased expression of FoxP3, NOS1, and CSF1R.
Together, these data suggested that THC-mediated alterations in miR expression in the lungs may play a critical role in the induction of immunosuppressive Tregs and MDSCs as well as suppression of cytokine storm leading to attenuation of SEB-mediated lung injury.”
https://pubmed.ncbi.nlm.nih.gov/32612530/
“In summary, the current study suggests that treatment of mice with THC post-SEB challenge protects mice from SEB-mediated toxicity by inhibiting inflammation and ARDS through the modulation of miRs. Because SEB is a super antigen that drives cytokine storm, our studies suggest that THC is a potent anti-inflammatory agent that has the potential to be used as a therapeutic modality to treat SEB-induced ARDS.
It is of interest to note that a significant proportion of Coronavirus disease 2019 (COVID-19) patients come down with sepsis and ARDS accompanied by cytokine storm. ”
https://www.frontiersin.org/articles/10.3389/fphar.2020.00893/full
 “Objectives: To investigate the action of cannabinoids on spasticity and pain in secondary progressive multiple sclerosis, by means of neurophysiological indexes.
“Objectives: To investigate the action of cannabinoids on spasticity and pain in secondary progressive multiple sclerosis, by means of neurophysiological indexes.
 “Astrocytomas, the most prevalent primary brain tumors, can be divided by histology and malignancy levels into four following types: pilocytic astrocytoma (grade I), diffuse fibrillary astrocytoma (grade II), anaplastic astrocytoma (grade III), and glioblastoma multiforme (grade IV). For high grade astrocytomas (grade III and grade IV), blood vessels formation is considered as the most important property.
“Astrocytomas, the most prevalent primary brain tumors, can be divided by histology and malignancy levels into four following types: pilocytic astrocytoma (grade I), diffuse fibrillary astrocytoma (grade II), anaplastic astrocytoma (grade III), and glioblastoma multiforme (grade IV). For high grade astrocytomas (grade III and grade IV), blood vessels formation is considered as the most important property. “Hempseeds, the edible fruits of the Cannabis sativa L. plant, were initially considered a by-product of the hemp technical fibre industry. Nowadays, following the restorationing of the cultivation of C. sativa L. plants containing an amount of delta-9-tetrahydrocannabinol (THC) <0.3% or 0.2% (industrial hemp) there is a growing interest for the hempseeds production due to their high nutritional value and functional features.
“Hempseeds, the edible fruits of the Cannabis sativa L. plant, were initially considered a by-product of the hemp technical fibre industry. Nowadays, following the restorationing of the cultivation of C. sativa L. plants containing an amount of delta-9-tetrahydrocannabinol (THC) <0.3% or 0.2% (industrial hemp) there is a growing interest for the hempseeds production due to their high nutritional value and functional features.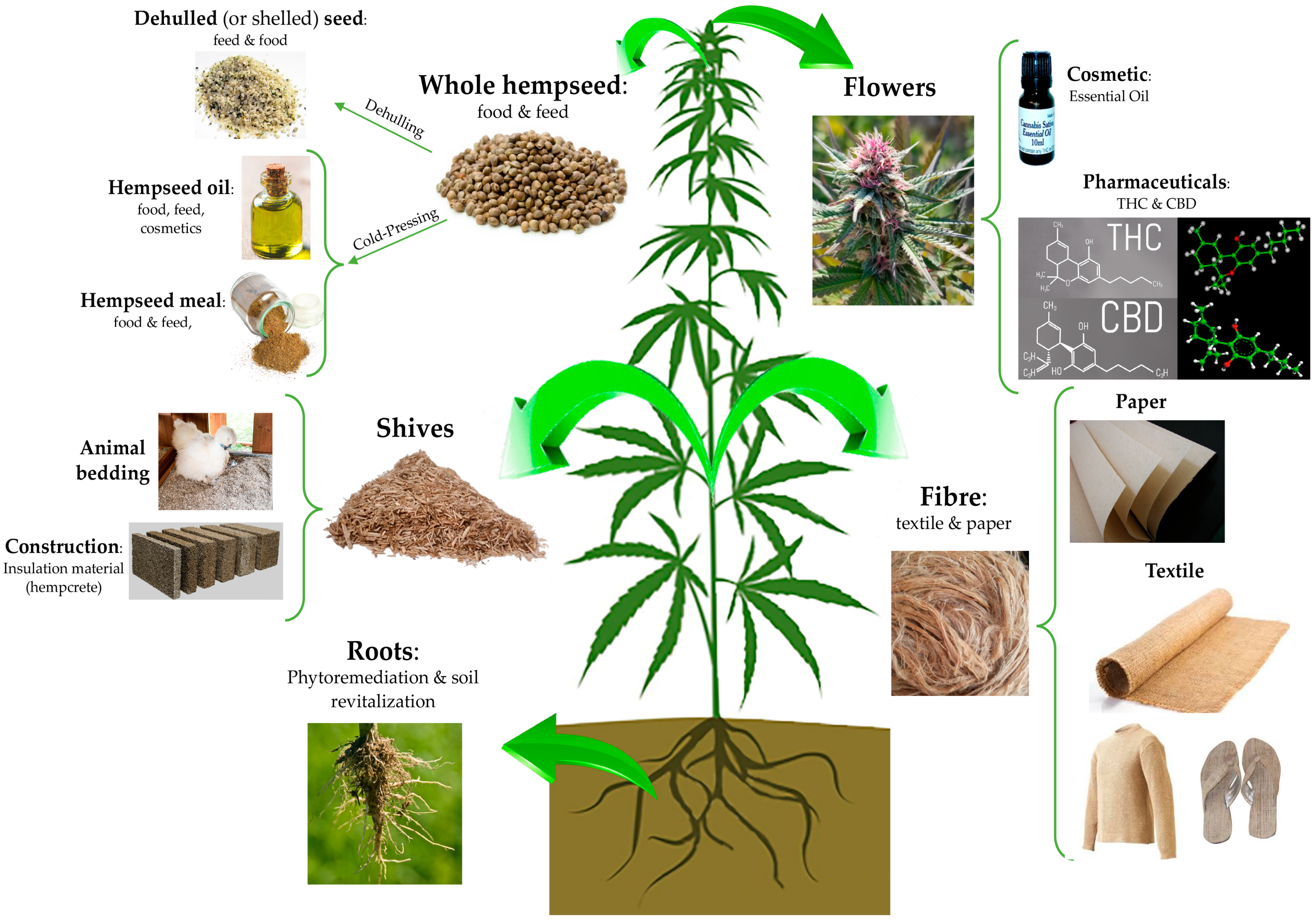
 “Acute Respiratory Distress Syndrome (ARDS) is a life-threatening complication that can ensue following Staphylococcus aureus infection. The enterotoxin produced by these bacteria (SEB) acts as a superantigen thereby activating a large proportion of T cells leading to cytokine storm and severe lung injury.
“Acute Respiratory Distress Syndrome (ARDS) is a life-threatening complication that can ensue following Staphylococcus aureus infection. The enterotoxin produced by these bacteria (SEB) acts as a superantigen thereby activating a large proportion of T cells leading to cytokine storm and severe lung injury. “Embase and Pubmed were systematically searched for articles addressing the neuroprotective properties of phytocannabinoids, aside from cannabidiol and Δ9 -tetrahydrocannabinol, including Δ9 -tetrahydrocannabinolic acid (Δ9 -THCA), Δ9 -tetrahydrocannabivarin (Δ9 -THCV), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabichromene (CBC), cannabichromenic acid (CBCA), cannabichromevarin (CBCV), cannabigerol (CBG), cannabigerolic acid (CBGA), cannabigerivarin (CBGV), cannabigerovarinic acid (CBGVA), cannabichromevarinic acid (CBCVA) cannabidivarinic acid (CBDVA) and cannabinol (CBN).
“Embase and Pubmed were systematically searched for articles addressing the neuroprotective properties of phytocannabinoids, aside from cannabidiol and Δ9 -tetrahydrocannabinol, including Δ9 -tetrahydrocannabinolic acid (Δ9 -THCA), Δ9 -tetrahydrocannabivarin (Δ9 -THCV), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabichromene (CBC), cannabichromenic acid (CBCA), cannabichromevarin (CBCV), cannabigerol (CBG), cannabigerolic acid (CBGA), cannabigerivarin (CBGV), cannabigerovarinic acid (CBGVA), cannabichromevarinic acid (CBCVA) cannabidivarinic acid (CBDVA) and cannabinol (CBN). “Precise cannabis treatment dosing remains a major challenge, leading to physicians’ reluctance to prescribe medical cannabis.
“Precise cannabis treatment dosing remains a major challenge, leading to physicians’ reluctance to prescribe medical cannabis. “Scientific research on how consumption of whole, natural Cannabis flower affects low mood and behavioral motivations more generally is largely nonexistent, and few studies to date have measured how common and commercially available Cannabis flower used in vivo may affect the experience of “depression” in real-time.
“Scientific research on how consumption of whole, natural Cannabis flower affects low mood and behavioral motivations more generally is largely nonexistent, and few studies to date have measured how common and commercially available Cannabis flower used in vivo may affect the experience of “depression” in real-time.