 “Clinical and preclinical evidence has indicated that estrogen depletion leads to memory impairments and increases the susceptibility to neural damage.
“Clinical and preclinical evidence has indicated that estrogen depletion leads to memory impairments and increases the susceptibility to neural damage.
Here, we have sought to investigate the effects of Cannabidiol (CBD) a non-psychotomimetic compound from Cannabis sativa, on memory deficits induced by estrogen depletion in rats, and its underlying mechanisms.
Results revealed that ovariectomy impaired avoidance memory, and CBD was able to completely reverse estrogen depletion-induced memory impairment. Ovariectomy also reduced Akt/GSK3β pathway’s activation by decreasing the phosphorylation levels of Akt and GSK3β and Bcl2 levels, which were ameliorated by CBD.
The present results indicate that CBD leads to a functional recovery accompanied by the Akt/GSK3β survival pathway’s activation, supporting its potential as a treatment for estrogen decline-induced deterioration of neural functioning and maintenance.”
“In the present study, we aimed to understand the possible neuroprotective effect of CBD against estrogen depletion-induced emotional memory deficits, using an animal model of ovariectomy-induced estrogen depletion. Once CBD and estradiol modulate a common pathway, we speculated whether CBD would be able to reverse the deleterious effect of estradiol decline observed in menopause. Results revealed that ovariectomy impaired avoidance memory, and CBD was able to completely reverse estrogen depletion-induced memory impairment.”
https://www.sciencedirect.com/science/article/abs/pii/S0166432821004435?via%3Dihub

 “The liver is a key metabolic organ that is particularly sensitive to environmental factors, including UV radiation. As UV radiation induces oxidative stress and inflammation, natural compounds are under investigation as one method to counteract these consequences.
“The liver is a key metabolic organ that is particularly sensitive to environmental factors, including UV radiation. As UV radiation induces oxidative stress and inflammation, natural compounds are under investigation as one method to counteract these consequences.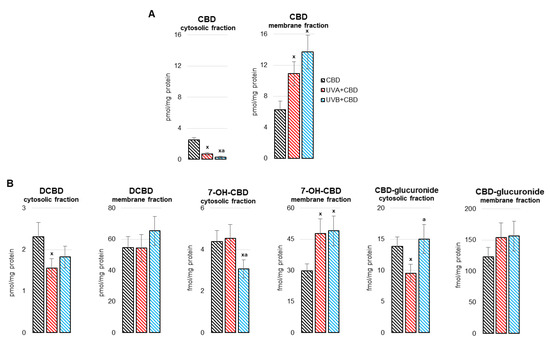
 “Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the loss of dopaminergic neurons in the Substantia Nigra pars compacta, leading to classical PD motor symptoms. Current therapies are purely symptomatic and do not modify disease progression.
“Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the loss of dopaminergic neurons in the Substantia Nigra pars compacta, leading to classical PD motor symptoms. Current therapies are purely symptomatic and do not modify disease progression.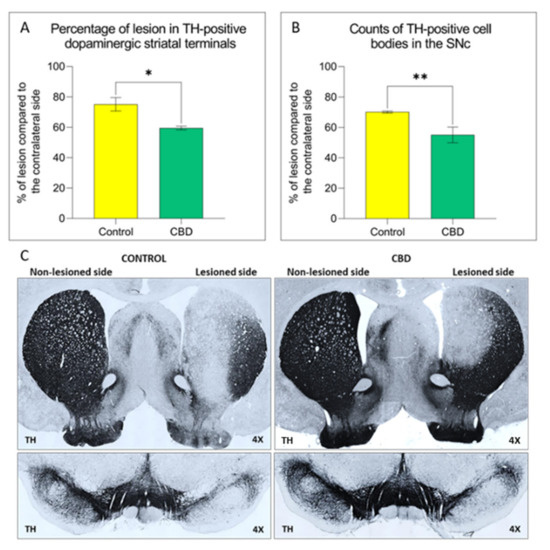
 “Obesity-related insulin resistance (IR) and attenuated brain insulin signaling are significant risk factors for neurodegenerative disorders, e.g., Alzheimer’s disease. IR and type 2 diabetes correlate with an increased concentration of sphingolipids, a class of lipids that play an essential structural role in cellular membranes and cell signaling pathways.
“Obesity-related insulin resistance (IR) and attenuated brain insulin signaling are significant risk factors for neurodegenerative disorders, e.g., Alzheimer’s disease. IR and type 2 diabetes correlate with an increased concentration of sphingolipids, a class of lipids that play an essential structural role in cellular membranes and cell signaling pathways. “Cannabidiol is increasingly considered for treatment of a wide range of medical conditions.
“Cannabidiol is increasingly considered for treatment of a wide range of medical conditions. 
 “Despite the high incidence of traumatic brain injury (TBI), there is no universal treatment to safely treat patients. Blunt brain injuries destroy primary neural tissue that results in impaired perfusion, excessive release of glutamate, inflammation, excitotoxicity, and progressive secondary neuronal cell death.
“Despite the high incidence of traumatic brain injury (TBI), there is no universal treatment to safely treat patients. Blunt brain injuries destroy primary neural tissue that results in impaired perfusion, excessive release of glutamate, inflammation, excitotoxicity, and progressive secondary neuronal cell death.  “Importance:
“Importance:  “Studies investigating the psychosomatic effects of social isolation in animals have shown that one of the physiologic system that gets disrupted by this environment-affective change is the Endocannabinoid System. As the levels of endocannabinoids change in limbic areas and prefrontal cortex during stressful times, so is the subject more prone to fearful and negative thoughts and aggressive behavior. The interplay of social isolation on the hypothalamic-pituitary-adrenal axis and cannabinoid tone triggers a vicious cycle which further impairs the natural body’s homeostatic neuroendocrine levels and provokes a series of risk factors for developing health complications. In this paper, we explore the psychosomatic impact of prolonged quarantine in healthy individuals, and propose management and coping strategies that may improve endocannabinoid tone, such as integration of probiotics, cannabidiol, meditation, and physical exercise interventions with the aim of supporting interpersonal, individual, and professional adherence with COVID-19 emergency public measures whilst minimizing their psycho-physical impact.”
“Studies investigating the psychosomatic effects of social isolation in animals have shown that one of the physiologic system that gets disrupted by this environment-affective change is the Endocannabinoid System. As the levels of endocannabinoids change in limbic areas and prefrontal cortex during stressful times, so is the subject more prone to fearful and negative thoughts and aggressive behavior. The interplay of social isolation on the hypothalamic-pituitary-adrenal axis and cannabinoid tone triggers a vicious cycle which further impairs the natural body’s homeostatic neuroendocrine levels and provokes a series of risk factors for developing health complications. In this paper, we explore the psychosomatic impact of prolonged quarantine in healthy individuals, and propose management and coping strategies that may improve endocannabinoid tone, such as integration of probiotics, cannabidiol, meditation, and physical exercise interventions with the aim of supporting interpersonal, individual, and professional adherence with COVID-19 emergency public measures whilst minimizing their psycho-physical impact.” “The Cannabis sativa plant has been used medicinally and recreationally for thousands of years, but recently only relatively some of its constituents have been identified.
“The Cannabis sativa plant has been used medicinally and recreationally for thousands of years, but recently only relatively some of its constituents have been identified.