“Delayed neurologic sequelae (DNS) are among the most serious complications of carbon monoxide (CO) poisoning caused partly by elevated neuroinflammation.
“Delayed neurologic sequelae (DNS) are among the most serious complications of carbon monoxide (CO) poisoning caused partly by elevated neuroinflammation.
WIN 55,212-2, a non-selective agonist of cannabinoid receptors, has been demonstrated to have anti-inflammatory properties in various brain disorders.
The anti-inflammatory action of WIN 55,212-2 is potentially associated with driving microglial M2 polarization. ST2 signaling is important in regulating inflammatory responses and microglial polarization. Therefore, we aimed to investigate the neuroprotective effect of WIN 55,212-2 on DNS after CO poisoning and elucidate its relationship with ST2-mediated microglial M2 polarization.
The behavioral tests showed that treatment with WIN 55,212-2 significantly ameliorates the cognitive impairment induced by CO poisoning.
This behavioral improvement was accompanied by reduced neuron loss, decreased production of pro-inflammatory cytokines, and a limited number of microglia in the hippocampus. Moreover, WIN 55,212-2 elevated the protein expression of IL-33 (the ligand of ST2) and ST2, increased the ratio of CD206-positive (M2 phenotype) and ST2-positive microglia, and augmented production of M2 microglia-associated cytokines in the hippocampus of CO-exposed rats.
Furthermore, we observed that the WIN 55,212-2-mediated increases in ST2 protein expression, CD206-positive and ST2-positive microglia, and microglia-associated cytokines were blocked by the cannabinoid receptor 2 (CB2R) antagonist AM630 but not by the cannabinoid receptor 1 (CB1R) antagonist AM251. In contrast, the WIN 55,212-2-induced upregulation of the IL-33 protein expression was inhibited by AM251 but not by AM630.
Altogether, these findings reveal cannabinoid receptors as promising therapeutic agents for CO poisoning and identify ST2 signaling-related microglial M2 polarization as a new mechanism of cannabinoid-induced neuroprotection.”
https://www.ncbi.nlm.nih.gov/pubmed/31732924
https://link.springer.com/article/10.1007%2Fs12031-019-01429-2


 “Stiff person syndrome (SPS) is a disorder characterized by fluctuating, progressive and painful spasms of the limbs, trunk and face. The condition is frequently associated with other diseases, including malignancies1. Up to 10% of SPS cases are paraneoplastic (PSPS) and occur with various types of cancer 2. SPS is thought to be immune-mediated, with up to 60% of patients demonstrating antibodies to glutamic acid decarboxylase (GAD), the rate-limiting enzyme for the production of the inhibitory neurotransmitter γ-aminobutyric acid (GABA).”
“Stiff person syndrome (SPS) is a disorder characterized by fluctuating, progressive and painful spasms of the limbs, trunk and face. The condition is frequently associated with other diseases, including malignancies1. Up to 10% of SPS cases are paraneoplastic (PSPS) and occur with various types of cancer 2. SPS is thought to be immune-mediated, with up to 60% of patients demonstrating antibodies to glutamic acid decarboxylase (GAD), the rate-limiting enzyme for the production of the inhibitory neurotransmitter γ-aminobutyric acid (GABA).”  “Ecological research suggests that increased access to
“Ecological research suggests that increased access to  “Autism Spectrum Disorders comprise conditions that may affect cognitive development, motor skills, social interaction, communication, and behavior. This set of functional deficits often results in lack of independence for the diagnosed individuals, and severe distress for patients, families, and caregivers.
“Autism Spectrum Disorders comprise conditions that may affect cognitive development, motor skills, social interaction, communication, and behavior. This set of functional deficits often results in lack of independence for the diagnosed individuals, and severe distress for patients, families, and caregivers. “This paper aimed to systematically examine the efficacy and adverse event (AE) profile of
“This paper aimed to systematically examine the efficacy and adverse event (AE) profile of  “This study sought to determine the prevalence, tolerability, and self-reported effectiveness of
“This study sought to determine the prevalence, tolerability, and self-reported effectiveness of  “The protective effect of
“The protective effect of  “Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder with no known cure and with an average life expectancy of 3-5 years post diagnosis.
“Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder with no known cure and with an average life expectancy of 3-5 years post diagnosis. “Cannabis sativa L. is one of the most-studied species for its phytochemistry due to the abundance of secondary metabolites, including
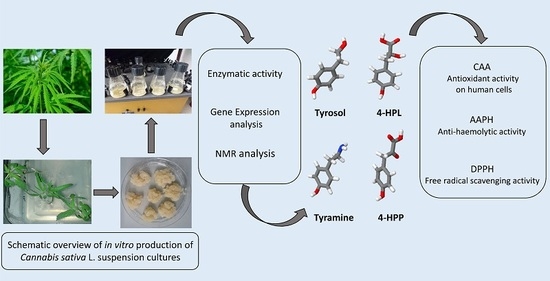
“Cannabis sativa L. is one of the most-studied species for its phytochemistry due to the abundance of secondary metabolites, including