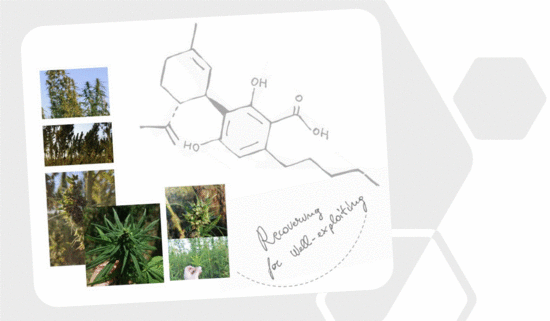
 “Cannabidiolic acid (CBDA) is the main phytocannabinoid in fiber and seed-oil hemp (Cannabis sativa L.) plants, but its potential health-related capabilities have been masked for years by a greater scientific interest towards its neutral derivative cannabidiol (CBD). This review aims to collect from the literature and critically discuss all the information about this molecule, starting from its biosynthesis, and focusing on its bioactivity, as an anti-inflammatory, anti-emetic, anti-convulsant, and anti-cancerogenic drug. Furthermore, in the awareness that, despite its multiple bioactive effects, currently poor efforts have been made to achieve its reliable purification, herein, we propose a relatively simple, fast, and inexpensive procedure for its recovery from pollen of industrial hemp cultivars. Spectroscopic and spectrometric techniques allowed us to unequivocally identify pure isolated CBDA and to distinguish it from the constitutional isomer tetrahydrocannabinolic acid (THCA-A).”
“Cannabidiolic acid (CBDA) is the main phytocannabinoid in fiber and seed-oil hemp (Cannabis sativa L.) plants, but its potential health-related capabilities have been masked for years by a greater scientific interest towards its neutral derivative cannabidiol (CBD). This review aims to collect from the literature and critically discuss all the information about this molecule, starting from its biosynthesis, and focusing on its bioactivity, as an anti-inflammatory, anti-emetic, anti-convulsant, and anti-cancerogenic drug. Furthermore, in the awareness that, despite its multiple bioactive effects, currently poor efforts have been made to achieve its reliable purification, herein, we propose a relatively simple, fast, and inexpensive procedure for its recovery from pollen of industrial hemp cultivars. Spectroscopic and spectrometric techniques allowed us to unequivocally identify pure isolated CBDA and to distinguish it from the constitutional isomer tetrahydrocannabinolic acid (THCA-A).”
https://pubmed.ncbi.nlm.nih.gov/32517131/
https://www.mdpi.com/1420-3049/25/11/2638

 “Identifying candidate drugs effective in the new coronavirus disease 2019 (Covid-19) is crucial,
“Identifying candidate drugs effective in the new coronavirus disease 2019 (Covid-19) is crucial,  “Obesity rates are increasing worldwide and there is a need for novel therapeutic treatment options.
“Obesity rates are increasing worldwide and there is a need for novel therapeutic treatment options. “A randomized double-blind clinical trial evaluating the efficacy of a medicinal cannabis formulation (ZTL-101; Zelira Therapeutics Ltd, Perth, Australia) for treating chronic insomnia showed that the therapy is effective and safe.
“A randomized double-blind clinical trial evaluating the efficacy of a medicinal cannabis formulation (ZTL-101; Zelira Therapeutics Ltd, Perth, Australia) for treating chronic insomnia showed that the therapy is effective and safe.
 “Cannabidiol (CBD) is a naturally occurring, non-psychotropic cannabinoid of the hemp plant Cannabis sativa L. and has been known to induce several physiological and pharmacological effects. While CBD is approved as a medicinal product subject to prescription, it is also widely sold over the counter (OTC) in the form of food supplements, cosmetics and electronic cigarette liquids. However, regulatory difficulties arise from its origin being a narcotic plant or its status as an unapproved novel food ingredient.
“Cannabidiol (CBD) is a naturally occurring, non-psychotropic cannabinoid of the hemp plant Cannabis sativa L. and has been known to induce several physiological and pharmacological effects. While CBD is approved as a medicinal product subject to prescription, it is also widely sold over the counter (OTC) in the form of food supplements, cosmetics and electronic cigarette liquids. However, regulatory difficulties arise from its origin being a narcotic plant or its status as an unapproved novel food ingredient. “Alzheimer’s Dementia (AD) is a devastating neurodegenerative disease that affects approximately 17% of people aged 75-84. Neuropsychiatric symptoms (NPS) such as delusions, agitation, anxiety, and hallucinations are present in up to 95% of patients in all stages of dementia. To date, any approved and effective pharmacological interventions for the treatment of NPS are still not available.
“Alzheimer’s Dementia (AD) is a devastating neurodegenerative disease that affects approximately 17% of people aged 75-84. Neuropsychiatric symptoms (NPS) such as delusions, agitation, anxiety, and hallucinations are present in up to 95% of patients in all stages of dementia. To date, any approved and effective pharmacological interventions for the treatment of NPS are still not available. “Rationale: When acutely administered intraperitoneally, the non-psychoactive cannabinoid cannabidiol (CBD), its acidic precursor cannabidiolic acid (CBDA) and a stable methyl ester of CBDA (HU-580) reduce lithium chloride (LiCl)-induced conditioned gaping in male rats (a selective preclinical model of acute nausea) via activation of the serotonin 1A (5-HT1A) receptor.
“Rationale: When acutely administered intraperitoneally, the non-psychoactive cannabinoid cannabidiol (CBD), its acidic precursor cannabidiolic acid (CBDA) and a stable methyl ester of CBDA (HU-580) reduce lithium chloride (LiCl)-induced conditioned gaping in male rats (a selective preclinical model of acute nausea) via activation of the serotonin 1A (5-HT1A) receptor. “The major phytocannabinoid cannabidiol (
“The major phytocannabinoid cannabidiol ( “Introduction: Severe Behavioural Problems (SBP) are a major contributor to morbidity in children with Intellectual Disability (ID). Medications used to treat SBP in ID are associated with a high risk of side effects. Cannabidiol has potential therapeutic effects in SBP. This pilot study aimed to investigate the feasibility of conducting a randomized placebo-controlled trial of cannabidiol to reduce SBP in children with ID.
“Introduction: Severe Behavioural Problems (SBP) are a major contributor to morbidity in children with Intellectual Disability (ID). Medications used to treat SBP in ID are associated with a high risk of side effects. Cannabidiol has potential therapeutic effects in SBP. This pilot study aimed to investigate the feasibility of conducting a randomized placebo-controlled trial of cannabidiol to reduce SBP in children with ID.