 “Cannabinoids such as ▵-9-THC and CBD can downregulate the immune response by modulating the endocannabinoid system. This modulation is relevant for the treatment of prevalent autoimmune diseases (ADs), such as multiple sclerosis (MS), systemic lupus erythematosus (SLE), diabetes mellitus type 1 (DMT1), and rheumatoid arthritis (RA). These conditions require new therapeutic options with fewer side effects for the control of the autoimmune response. Objective: to conduct a literature review of preclinical scientific evidence that supports further clinical investigations for the use of cannabinoids (natural or synthetic) as potential immunomodulators of the immune response in ADs.
“Cannabinoids such as ▵-9-THC and CBD can downregulate the immune response by modulating the endocannabinoid system. This modulation is relevant for the treatment of prevalent autoimmune diseases (ADs), such as multiple sclerosis (MS), systemic lupus erythematosus (SLE), diabetes mellitus type 1 (DMT1), and rheumatoid arthritis (RA). These conditions require new therapeutic options with fewer side effects for the control of the autoimmune response. Objective: to conduct a literature review of preclinical scientific evidence that supports further clinical investigations for the use of cannabinoids (natural or synthetic) as potential immunomodulators of the immune response in ADs.
Methodology: A systematic search was carried out in different databases using different MeSH terms, such as Cannabis sativa L., cannabinoids, immunomodulation, and ADs. Initially, 677 journal articles were found. After filtering by publication date (from 2000 to 2020 for SLE, DMT1, and RA; and 2010 to 2020 for MS) and removing the duplicate items, 200 articles were selected and analyzed by title and summary associated with the use of cannabinoids as immunomodulatory treatment for those diseases.
Results: Evidence of the immunomodulatory effect of cannabinoids in the diseases previously mentioned, but SLE that did not meet the search criteria, was summarized from 24 journal articles. CBD was found to be one of the main modulators of the immune response. This molecule decreased the number of Th1 and Th17 proinflammatory cells and the production of the proinflammatory cytokines, interleukin (IL)-1, IL-12, IL-17, interferon (IFN)-γ, and tumor necrosis factor alpha, in mouse models of MS and DMT1. Additionally, new synthetic cannabinoid-like molecules, with agonist or antagonist activity on CB1, CB2, TRPV1, PPAR-α, and PPAR-γ receptors, have shown anti-inflammatory properties in MS, DMT1, and RA.
Conclusion: Data from experimental animal models of AD showed that natural and synthetic cannabinoids downregulate inflammatory responses mediated by immune cells responsible for AD chronicity and progression. Although synthetic cannabinoid-like molecules were evaluated in just two clinical trials, they corroborated the potential use of cannabinoids to treat some ADs. Notwithstanding, new cannabinoid-based approaches are required to provide alternative treatments to patients affected by the large group of ADs.”
https://pubmed.ncbi.nlm.nih.gov/34030476/
https://www.liebertpub.com/doi/10.1089/can.2020.0183

 “Many people with MS (pwMS) use unregulated cannabis or cannabis products to treat the symptoms associated with the disease. In line with this, Sativex, a synthetic combination of cannabidiol (CBD) and Δ9-tetrahydrocannabinol (Δ9-THC) has been approved to treat symptoms of spasticity.
“Many people with MS (pwMS) use unregulated cannabis or cannabis products to treat the symptoms associated with the disease. In line with this, Sativex, a synthetic combination of cannabidiol (CBD) and Δ9-tetrahydrocannabinol (Δ9-THC) has been approved to treat symptoms of spasticity.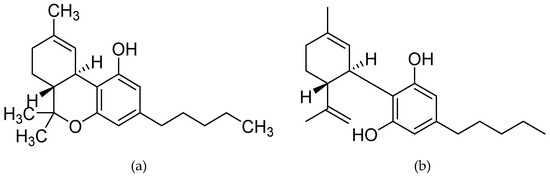
 “Δ9 -Tetrahydrocannabinol (THC), the main bioactive compound found in the plant Cannabis sativa, exerts its effects by activating cannabinoid receptors present in many neural cells.
“Δ9 -Tetrahydrocannabinol (THC), the main bioactive compound found in the plant Cannabis sativa, exerts its effects by activating cannabinoid receptors present in many neural cells. “Psoriasis is associated with increased production of reactive oxygen species which leads to oxidative stress.
“Psoriasis is associated with increased production of reactive oxygen species which leads to oxidative stress.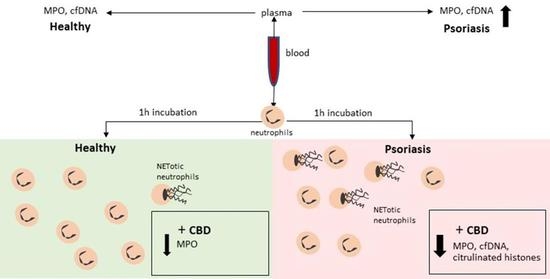
 “The immune-suppressive effects of cannabidiol (CBD) are attributed to the modulation of essential immunological signaling pathways and receptors. Mechanistic understanding of the pharmacological effects of CBD emphasizes the therapeutic potential of CBD as a novel immune modulator.
“The immune-suppressive effects of cannabidiol (CBD) are attributed to the modulation of essential immunological signaling pathways and receptors. Mechanistic understanding of the pharmacological effects of CBD emphasizes the therapeutic potential of CBD as a novel immune modulator. “HIV/SIV-associated oral mucosal disease/dysfunction (HAOMD) (gingivitis/periodontitis/salivary adenitis) represents a major comorbidity affecting HIV patients on anti-retroviral therapy.
“HIV/SIV-associated oral mucosal disease/dysfunction (HAOMD) (gingivitis/periodontitis/salivary adenitis) represents a major comorbidity affecting HIV patients on anti-retroviral therapy.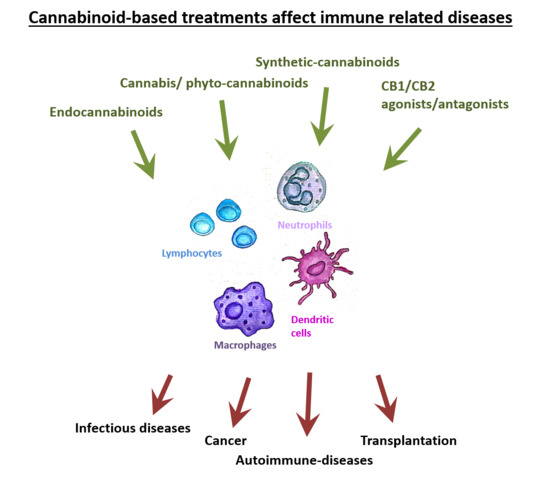
 “Painful tonic spasm (PTS) is a common yet debilitating symptom in patients with neuromyelitis optica spectrum disorder (NMOSD), especially those with longitudinally extensive transverse myelitis. Although carbamazepine is an effective treatment, it poses the risk of severe adverse reactions, such as Steven-Johnson syndrome (SJS).
“Painful tonic spasm (PTS) is a common yet debilitating symptom in patients with neuromyelitis optica spectrum disorder (NMOSD), especially those with longitudinally extensive transverse myelitis. Although carbamazepine is an effective treatment, it poses the risk of severe adverse reactions, such as Steven-Johnson syndrome (SJS). “Moderate to severe spasticity is commonly reported in Multiple Sclerosis (MS) and its management is still a challenge. Cannabinoids were recently suggested as add-on therapy for the treatment of spasticity and chronic pain in MS but there is no conclusive scientific evidence on their safety, especially on cognition and over long periods.
“Moderate to severe spasticity is commonly reported in Multiple Sclerosis (MS) and its management is still a challenge. Cannabinoids were recently suggested as add-on therapy for the treatment of spasticity and chronic pain in MS but there is no conclusive scientific evidence on their safety, especially on cognition and over long periods. “Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system, affecting ambulation even in people with only mild neurological signs. Patients with MS frequently experience spasticity, which contributes significantly to impair their motor functions, including ambulation, owing to muscle stiffness, spasms, and pain.
“Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system, affecting ambulation even in people with only mild neurological signs. Patients with MS frequently experience spasticity, which contributes significantly to impair their motor functions, including ambulation, owing to muscle stiffness, spasms, and pain.