
“Glioblastoma (GBM) is the most frequent malignant tumor of the central nervous system in humans with a median survival time of less than 15 months.
∆9-Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best-characterized components of Cannabis sativa plants with modulating effects on cannabinoid receptors 1 and 2 (CB1 and CB2) and on orphan receptors such as GPR18 or GPR55. Previous studies have demonstrated anti-tumorigenic effects of THC and CBD in several tumor entities including GBM, mostly mediated via CB1 or CB2.
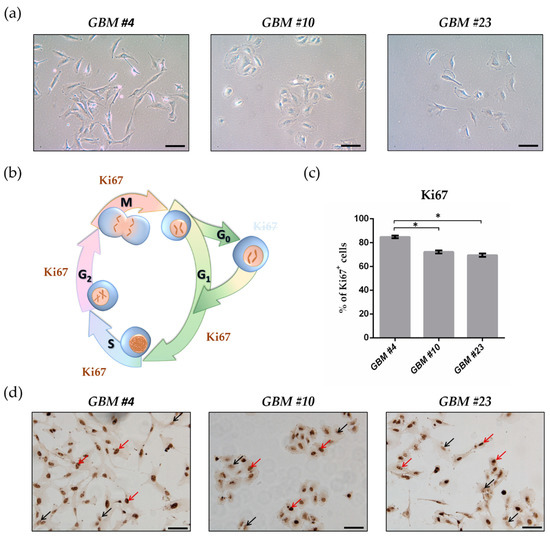
In this study, we investigated the non-CB1/CB2 effects of THC on the cell cycle of GBM cells isolated from human tumor samples.
Cell cycle entry was measured after 24 h upon exposure by immunocytochemical analysis of Ki67 as proliferation marker. The Ki67-reducing effect of THC was abolished in the presence of CBD, whereas CBD alone did not cause any changes. To identify the responsible receptor for THC effects, we first characterized the cells regarding their expression of different cannabinoid receptors: CB1, CB2, GPR18, and GPR55. Secondly, the receptors were pharmacologically blocked by application of their selective antagonists AM281, AM630, O-1918, and CID16020046 (CID), respectively. All examined cells expressed the receptors, but only in presence of the GPR55 antagonist CID was the THC effect diminished. Stimulation with the GPR55 agonist lysophosphatidylinositol (LPI) revealed similar effects as obtained for THC. The LPI effects were also inhibited by CBD and CID, confirming a participation of GPR55 and suggesting its involvement in modifying the cell cycle of patient-derived GBM cells.”
https://pubmed.ncbi.nlm.nih.gov/33802282/
https://www.mdpi.com/2072-6694/13/5/1064


 “Cannabis was extensively utilized for its medicinal properties till the 19th century. A steep decline in its medicinal usage was observed later due to its emergence as an illegal recreational drug. Advances in technology and scientific findings led to the discovery of delta-9-tetrahydrocannabinol (THC), the primary psychoactive compound of cannabis, that further led to the discovery of endogenous cannabinoids system consisting of G-protein-coupled receptors – cannabinoid receptor 1 and cannabinoid receptor 2 along with their ligands, mainly anandamide and 2-arachidonoylglycerol. Endocannabinoid (EC) is shown to be a modulator not only for physiological functions but also for the immune system, endocrine network, and central nervous system. Medicinal research and meta-data analysis over the last few decades have shown a significant potential for both THC and cannabidiol (CBD) to exert palliative effects. People suffering from many forms of advanced stages of cancers undergo chemotherapy-induced nausea and vomiting followed by severe and chronic neuropathic pain and weight loss. THC and CBD exhibit effective analgesic, anxiolytic, and appetite-stimulating effect on patients suffering from cancer. Drugs currently available in the market to treat such chemotherapy-induced cancer-related ailments are Sativex (GW Pharmaceutical), Dronabinol (Unimed Pharmaceuticals), and Nabilone (Valeant Pharmaceuticals). Apart from exerting palliative effects, THC also shows promising role in the treatment of cancer growth, neurodegenerative diseases (multiple sclerosis and Alzheimer’s disease), and alcohol addiction and hence should be exploited for potential benefits. The current review discusses the nature and role of CB receptors, specific applications of cannabinoids, and major studies that have assessed the role of cannabinoids in cancer management.”
“Cannabis was extensively utilized for its medicinal properties till the 19th century. A steep decline in its medicinal usage was observed later due to its emergence as an illegal recreational drug. Advances in technology and scientific findings led to the discovery of delta-9-tetrahydrocannabinol (THC), the primary psychoactive compound of cannabis, that further led to the discovery of endogenous cannabinoids system consisting of G-protein-coupled receptors – cannabinoid receptor 1 and cannabinoid receptor 2 along with their ligands, mainly anandamide and 2-arachidonoylglycerol. Endocannabinoid (EC) is shown to be a modulator not only for physiological functions but also for the immune system, endocrine network, and central nervous system. Medicinal research and meta-data analysis over the last few decades have shown a significant potential for both THC and cannabidiol (CBD) to exert palliative effects. People suffering from many forms of advanced stages of cancers undergo chemotherapy-induced nausea and vomiting followed by severe and chronic neuropathic pain and weight loss. THC and CBD exhibit effective analgesic, anxiolytic, and appetite-stimulating effect on patients suffering from cancer. Drugs currently available in the market to treat such chemotherapy-induced cancer-related ailments are Sativex (GW Pharmaceutical), Dronabinol (Unimed Pharmaceuticals), and Nabilone (Valeant Pharmaceuticals). Apart from exerting palliative effects, THC also shows promising role in the treatment of cancer growth, neurodegenerative diseases (multiple sclerosis and Alzheimer’s disease), and alcohol addiction and hence should be exploited for potential benefits. The current review discusses the nature and role of CB receptors, specific applications of cannabinoids, and major studies that have assessed the role of cannabinoids in cancer management.” “The main aspects of severe COVID-19 disease pathogenesis include hyper-induction of proinflammatory cytokines, also known as ‘cytokine storm’, that precedes acute respiratory distress syndrome (ARDS) and often leads to death. COVID-19 patients often suffer from lung fibrosis, a serious and untreatable condition. There remains no effective treatment for these complications.
“The main aspects of severe COVID-19 disease pathogenesis include hyper-induction of proinflammatory cytokines, also known as ‘cytokine storm’, that precedes acute respiratory distress syndrome (ARDS) and often leads to death. COVID-19 patients often suffer from lung fibrosis, a serious and untreatable condition. There remains no effective treatment for these complications. “Inflammasomes are cytoplasmic inflammatory signaling protein complexes that detect microbial materials, sterile inflammatory insults, and certain host-derived elements. Inflammasomes, once activated, promote caspase-1-mediated maturation and secretion of pro-inflammatory cytokines, interleukin (IL)-1β and IL-18, leading to pyroptosis. Current advances in inflammasome research support their involvement in the development of chronic inflammatory disorders in contrast to their role in regulating innate immunity.
“Inflammasomes are cytoplasmic inflammatory signaling protein complexes that detect microbial materials, sterile inflammatory insults, and certain host-derived elements. Inflammasomes, once activated, promote caspase-1-mediated maturation and secretion of pro-inflammatory cytokines, interleukin (IL)-1β and IL-18, leading to pyroptosis. Current advances in inflammasome research support their involvement in the development of chronic inflammatory disorders in contrast to their role in regulating innate immunity. “Preclinical data suggest some cannabinoids may exert antitumour effects against glioblastoma (GBM). Safety and preliminary efficacy of nabiximols oromucosal cannabinoid spray plus dose-intense temozolomide (DIT) was evaluated in patients with first recurrence of GBM.
“Preclinical data suggest some cannabinoids may exert antitumour effects against glioblastoma (GBM). Safety and preliminary efficacy of nabiximols oromucosal cannabinoid spray plus dose-intense temozolomide (DIT) was evaluated in patients with first recurrence of GBM. “Cannabis sativa contains more than 500 constituents, yet the anticancer properties of the vast majority of cannabis compounds remains unknown. We aimed to identify cannabis compounds and their combinations presenting cytotoxicity against bladder urothelial carcinoma (UC), the most common urinary system cancer.
“Cannabis sativa contains more than 500 constituents, yet the anticancer properties of the vast majority of cannabis compounds remains unknown. We aimed to identify cannabis compounds and their combinations presenting cytotoxicity against bladder urothelial carcinoma (UC), the most common urinary system cancer.

 “Plant-based therapies date back centuries. Cannabis sativa is one such plant that was used medicinally up until the early part of the 20th century.
“Plant-based therapies date back centuries. Cannabis sativa is one such plant that was used medicinally up until the early part of the 20th century.